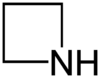
Chemistry:Azetidine
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azetidine[1] | |||
| Systematic IUPAC name
Azacyclobutane | |||
| Other names
Azetane
Trimethylene imine 1,3-Propylenimine | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 102384 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| 986 | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C3H7N | |||
| Molar mass | 57.09 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 0.847 g/cm3 at 25 °C | ||
| Boiling point | 61 to 62 °C (142 to 144 °F; 334 to 335 K) | ||
| miscible | |||
| Hazards | |||
| Main hazards | Somewhat strong base, combustible | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H225, H314 | |||
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P403+235, P405, P501 | |||
| Related compounds | |||
Other anions
|
Oxetane, Phosphetane, Thietane | ||
Related compounds
|
Aziridine, Diazetidine, Pyrrolidine, Piperidine, Azepane, Azocane, Azonane | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Azetidine is a saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines.
Synthesis and occurrence
Azetidines can be prepared by reduction of azetidinones (β-lactams) with lithium aluminium hydride. Even more effective is a mixture of lithium aluminium hydride and aluminium trichloride, a source of "AlClH2" and "AlCl2H".[2] Azetidine can also be produced by a multistep route from 3-amino-1-propanol.[3]
Regio- and diastereoselective synthesis of 2-arylazetidines could be performed from appropriately substituted oxiranes via ring transformation. It is controlled by Baldwin's Rules with remarkable functional group tolerance. [citation needed]
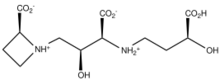
Azetidine and its derivatives are relatively rare structural motifs in natural products. They are a component of mugineic acids and penaresidins. Perhaps the most abundant azetidine containing natural product is azetidine-2-carboxylic acid - a toxic mimic of proline.[4]
See also
- Azete, the unsaturated analog
References
- ↑ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Alcaide, Benito; Almendros, Pedro; Aragoncillo, Cristina (2007). "Β-Lactams: Versatile Building Blocks for the Stereoselective Synthesis of Non-β-Lactam Products". Chemical Reviews 107 (11): 4437–4492. doi:10.1021/cr0307300. PMID 17649981.
- ↑ Donald H. Wadsworth (1973). "Azetidine". Organic Syntheses 53: 13. doi:10.15227/orgsyn.053.0013.
- ↑ Kovács, Ervin; Ferenc, Faigl; Zoltan, Mucsi (Aug 10, 2020). "Regio- and Diastereoselective Synthesis of 2-Arylazetidines. Quantum Chemical Explanation of Baldwin's Rules for the Ring-formation Reactions of Oxiranes.". Journal of Organic Chemistry 85 (17): 11226–11239. doi:10.1021/acs.joc.0c01310. PMID 32786621.
External links
 |