Chemistry:Benzoylacetone
From HandWiki
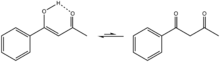
| |
| Names | |
|---|---|
| IUPAC name
1-phenylbutane-1,3-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H10O2 | |
| Molar mass | 162.188 g·mol−1 |
| Density | 1.0599 g/cm3 |
| Melting point | 56 °C (133 °F; 329 K) |
| Boiling point | 261.5 °C (502.7 °F; 534.6 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzoylacetone is the organic compound with the nominal formula C6H5C(O)CH2C(O)CH3. As a 1,3-dicarbonyl, it is a precursor to many heterocycles, such as pyrazoles.[1] It exists predominantly as the enol tautomer C6H5C(OH)=CHC(O)CH3.[2] Its conjugate base (pKa=8.7) forms stable complexes with transition metals and lanthanides.[3]
References
- ↑ Penning, Thomas D.; Talley, John J.; Bertenshaw, Stephen R.; Carter, Jeffery S.; Collins, Paul W.; Docter, Stephen; Graneto, Matthew J.; Lee, Len F. et al. (1997). "Synthesis and Biological Evaluation of the 1,5-Diarylpyrazole Class of Cyclooxygenase-2 Inhibitors: Identification of 4-[5-(4-Methylphenyl)-3- (Trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, Celecoxib)". Journal of Medicinal Chemistry 40 (9): 1347–1365. doi:10.1021/JM960803Q. PMID 9135032.
- ↑ Jones, R. D. G. (1976). "The crystal and molecular structure of the enol form of 1-phenyl-1,3-butanedione (Benzoylacetone) by neutron diffraction". Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry 32 (7): 2133–2136. doi:10.1107/S0567740876007267.
- ↑ McGehee, M. D.; Bergstedt, T.; Zhang, C.; Saab, A. P.; o'Regan, M. B.; Bazan, G. C.; Srdanov, V. I.; Heeger, A. J. (1999). "Narrow Bandwidth Luminescence from Blends with Energy Transfer from Semiconducting Conjugated Polymers to Europium Complexes". Advanced Materials 11 (16): 1349–1354. doi:10.1002/(SICI)1521-4095(199911)11:16<1349::AID-ADMA1349>3.0.CO;2-W.
 |

