Chemistry:Beryllium chromate
From HandWiki
 | |
| Identifiers | |
|---|---|
| Properties | |
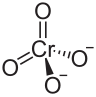
| BeCrO4 | |
| Molar mass | 125.0076 |
| Related compounds | |
Other cations
|
Magnesium chromate, Calcium chromate, Strontium chromate, Barium chromate, Radium chromate |
Related compounds
|
Beryllium chromite |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Beryllium chromate is a hypothetical inorganic compound, with the chemical formula of BeCrO4.[citation needed] It is predicted to have a certain bonding ability with noble gases.[1] Little evidence has been published supporting the existence of this material.
Claims
Beryllium chromate is claimed to be obtained from the reaction of beryllium hydroxide and chromium trioxide:[2]
- Be(OH)2 + CrO3 → BeCrO4 + H2O
The reaction of potassium chromate and beryllium sulfate is claimed to produce beryllium hydroxide:[3]
- BeSO4 + 2K2CrO4 + H2O → K2Cr2O7 + K2SO4 + Be(OH)2
References
- ↑ Pan, Sudip; Ghara, Manas; Ghosh, Sreyan; Chattaraj, Pratim K. (2016). "Noble gas bound beryllium chromate and beryllium hydrogen phosphate: a comparison with noble gas bound beryllium oxide" (in en). RSC Advances 6 (95): 92786–92794. doi:10.1039/C6RA20232B. ISSN 2046-2069. Bibcode: 2016RSCAd...692786P. http://xlink.rsc.org/?DOI=C6RA20232B.
- ↑ Bleyer, B.; Moormann, A. (1912-06-11). "Über Berylliumchromate" (in en). Zeitschrift für anorganische Chemie 76 (1): 70–78. doi:10.1002/zaac.19120760105. https://onlinelibrary.wiley.com/doi/10.1002/zaac.19120760105.
- ↑ Orlow, N. A. (1912-01-16). "Über Berylliumchromate" (in en). Zeitschrift für anorganische Chemie 79 (1): 365–367. doi:10.1002/zaac.19120790128. https://onlinelibrary.wiley.com/doi/10.1002/zaac.19120790128.
category:Beryllium compounds category:Chromates
 |

