Chemistry:Magnesium chromate
| |
| Names | |
|---|---|
| Other names
Magnesium chromate(VI)
Magnesium monochromate Magnesium monochromate(VI) | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
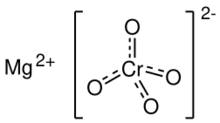
| MgCrO4 | |
| Molar mass | 140.297 g·mol−1 |
| Appearance | Yellow solid |
| soluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium chromate is a chemical compound, with the formula MgCrO4. It is a yellow, odorless, water-soluble salt with several important industrial uses. This chromate can be manufactured as a powder.
History
Before 1940, the literature about magnesium chromate and its hydrates was sparse, but studies starting in that year looked at its properties and solubility.[1]
Uses
It is available commercially in a variety of powders, from nanoscale to micron-sized, either as an anhydrous or hydrated form.[2][3]
As a hydrate, it is useful as a corrosion inhibitor and pigment,[4] or as an ingredient in cosmetics.[5] In 2011, an undecahydrate (containing 11 molecules of water) of this compound was discovered by scientists at the University College London.[6]
Hazards
Magnesium chromate hydrate should be stored at room temperature, and there is no current therapeutic use.[7] It is a confirmed carcinogen, and can cause acute dermititis, and possibly kidney and liver damage if inhaled, so it should be treated as a hazardous waste.[8]
References
- ↑ Hill, Arthur E.; Soth, Glenn C.; Ricci, John E. (1940). "The Systems Magnesium Chromate—Water and Ammonium Chromate—Water from 0 to 75 °C". Journal of the American Chemical Society 62 (8): 2131. doi:10.1021/ja01865a059.
- ↑ "Magnesium Chromate". American Elements. http://www.americanelements.com/mgcrat.html. Retrieved 2013-07-16.
- ↑ Li, Su Ping; Jia, Xiao Lin; Qi, Ya Fang (2011). "Synthesis of Nano-Crystalline Magnesium Chromate Spinel by Citrate Sol-Gel Method". Advanced Materials Research 284-286: 730. doi:10.4028/www.scientific.net/AMR.284-286.730.
- ↑ "Magnesium chromate hydrate, 99.8% (metals basis)". Us.vwr.com. https://us.vwr.com/store/catalog/product.jsp?product_id=9880222. Retrieved 2013-07-16.
- ↑ "Item # MG-401, Heavy Magnesium Chromate Powder On Atlantic Equipment Engineers, A Division Of Micron Metals, Inc". Metal-powders-compounds.micronmetals.com. http://metal-powders-compounds.micronmetals.com/item/high-purity-metal-powders-compounds/magnesium-chromate/mg-401. Retrieved 2013-07-16.
- ↑ Fortes, A. Dominic; Wood, Ian G. (March 2012). "X-ray powder diffraction analysis of a new magnesium chromate hydrate, MgCrO4·11H2O". Powder Diffraction 27 (1): 8–11. doi:10.1017/S088571561200005X. Bibcode: 2012PDiff..27....8F.
- ↑ "Magnesium chromate hydrate | CAS 23371-94-0 | Santa Cruz Biotech". Santa Cruz Biotechnology, Inc.. https://www.scbt.com/scbt/product/magnesium-chromate-hydrate-23371-94-0. Retrieved 2013-07-16.
- ↑ "Material Data Sheet". McGean-Rohco, Inc.. http://www.mcgean.com/documents/msds/usa-all/MS35500.pdf. Retrieved 2013-07-16.
 |

