Chemistry:Biuret

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Imidodicarbonic diamide[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 1703510 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 49702 | |
| KEGG | |
| MeSH | Biuret |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| HN(CONH 2) 2 | |
| Molar mass | 103.081 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| Density | 1.467 g/cm3 |
| Melting point | 190 °C (decomposes) |
| Thermochemistry | |
Heat capacity (C)
|
131.3 J/(mol·K) |
Std molar
entropy (S |
146.1 J/(mol·K) |
Std enthalpy of
formation (ΔfH⦵298) |
(−565.8) – (−561.6) kJ/mol |
Std enthalpy of
combustion (ΔcH⦵298) |
(−940.1) – (−935.9) kJ/mol |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | WARNING |
| H315, H319, H335 | |
| P261, P305+351+338 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
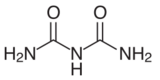
Biuret is a chemical compound with the chemical formula HN(CONH
2)
2. It is a white solid that is soluble in hot water. A variety of organic derivatives are known. The term "biuret" also describes a family of organic compounds with the chemical formula R1
R2
N–C(=O)–N(R3
)–C(=O)–NR4
R5
, where R1
, R2
, R3
, R4
and R5
are hydrogen, organyl or other groups. Also known as carbamylurea, it results from the condensation of two equivalents of urea. It is a common undesirable impurity in urea-based fertilizers, as biuret is toxic to plants.
Preparation and structure
The parent compound can be prepared by heating urea at 150 °C for ~6 hours until it gets slightly cloudy, then recrystallizing from water. After that, it can be recrystallized repeatedly from 2% sodium hydroxide solution and water to finally get base-free crystalline needles of the monohydrate which are free of cyanuric acid. While heating, a lot of ammonia is expelled:[2]
- 2 CO(NH
2)
2 → HN(CONH
2)
2 + NH
3
Under related conditions, pyrolysis of urea affords triuret O=C(–N(H)–C(=O)–NH
2)
2.[2]
In general, organic biurets (those with alkyl or aryl groups in place of one or more H atoms) are prepared by trimerization of isocyanates. For example, the trimer of 1,6-hexamethylene diisocyanate is also known as HDI-biuret.
In the anhydrous form, the molecule is planar and unsymmetrical in the solid state owing to intramolecular hydrogen bonding. The terminal C–N distances of 1.327 and 1.334 Å are shorter than the internal C–N distances of 1.379 and 1.391 Å. The C=O bond distances 1.247 and 1.237 Å. It crystallizes from water as the monohydrate.[3] 220 px|thumb|left|Structure of biuret in the solid state (blue = N, red = O, gray = C, cyan = H).
Applications
Biuret is also used as a non-protein nitrogen source in ruminant feed,[4] where it is converted into protein by gut microorganisms.[5] It is less favored than urea, due to its higher cost and lower digestibility[6] but the latter characteristic also slows down its digestion and so decreases the risk of ammonia toxicity.[6][7]
Biuret test
The biuret test is a chemical test for proteins and polypeptides. It is based on the biuret reagent, a blue solution that turns violet upon contact with proteins, or any substance with peptide bonds. The test and reagent do not actually contain biuret; they are so named because both biuret and proteins have the same response to the test.
History
Biuret was first prepared and studied by Gustav Heinrich Wiedemann (1826–1899) for his doctoral dissertation, which was submitted in 1847. His findings were reported in several articles.[8][9][10][11]
Related compounds
- Cyanuric acid
- Allophanic acid, the carboxylic acid derivative of biuret
References
- ↑ Favre, Henri A.; Powell, Warren H. (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. p. 866. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 Meessen, J. H.; Petersen, H.. "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_333.
- ↑ E. W. Hughes; H. Yakel; H. C. Freeman (1961). "The Crystal Structure of Biuret Hydrate". Acta Crystallogr. 14 (4): 345–352. doi:10.1107/S0365110X61001194.
- ↑ Beef cattle feed, Encyclopædia Britannica Online
- ↑ "Florida Cow-Calf Management, 2nd Edition - Feeding the Cow Herd". IFAS Extension, University of Florida. 2013. http://edis.ifas.ufl.edu/AN117.
- ↑ 6.0 6.1 Oltjen, R. R.; Williams, E. E.; Slyter, L. L.; Richardson, G. V. (1969). "Urea versus biuret in a roughage diet for steers". Journal of Animal Science 29 (5): 816–822. doi:10.2527/jas1969.295816x. PMID 5391979. http://www.journalofanimalscience.org/content/29/5/816. Retrieved 2013-10-22.
- ↑ Fonnesbeck, Paul V.; Kearl, Leonard C.; Harris, Lorin E. (1975). "Feed Grade Biuret as a Protein Replacement for Ruminants. A Review". Journal of Animal Science (Oxford University Press (OUP)) 40 (6): 1150–1184. doi:10.2527/jas1975.4061150x. ISSN 0021-8812. https://academic.oup.com/jas/article-abstract/40/6/1150/4699012.
- ↑ Wiedemann, G. (1848). "Ueber ein neues Zersetzungsproduct des Harnstoffs". Annalen der Physik 150 (5): 67–84. doi:10.1002/andp.18491500508. Bibcode: 1848AnP...150...67W. https://zenodo.org/record/1423618.
- ↑ Wiedemann, G. (1847). "Neues Zersetzungsproduct des Harnstoffs". Journal für Praktische Chemie 42 (3–4): 255–256. doi:10.1002/prac.18470420134. https://books.google.com/books?id=WNQPAAAAQAAJ&pg=PA255. This notice reports that biuret reacts with alkaline copper sulfate to produce a red solution – the so-called "Biuret test"
- ↑ Wiedemann, G. (1848). "Ueber eine neue, aus dem Harnstoff entstehende Verbindung". Journal für Praktische Chemie 43 (5): 271–280. doi:10.1002/prac.18480430133. https://books.google.com/books?id=hgYwAAAAIAAJ&pg=PA271.
- ↑ Wiedemann, G. (1848). "Biuret. Zersetzungsprodukt des Harnstoffs". Justus Liebig's Annalen der Chemie 68 (3): 323–326. doi:10.1002/jlac.18480680318.
 |
