Chemistry:Strictosidine
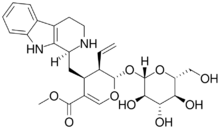
| |
| Names | |
|---|---|
| IUPAC name
Methyl (19S,20R)-19-(β-D-glucopyranosyloxy)-16,17,21,21a-tetradehydro-18-oxa-21a-homo-20,21-secoyohimban-16-carboxylate
| |
| Systematic IUPAC name
Methyl (4S,5R,6S)-5-ethenyl-4-{[(1S)-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl]methyl}-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-5,6-dihydro-4H-pyran-3-carboxylate | |
| Other names
Isovincoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C27H34N2O9 | |
| Molar mass | 530.574 g·mol−1 |
| Melting point | 193-197 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Strictosidine is a natural chemical compound and is classified as a glucoalkaloid and a vinca alkaloid. It is formed by the Pictet–Spengler condensation reaction of tryptamine with secologanin, catalyzed by the enzyme strictosidine synthase. Thousands of strictosidine derivatives are sometimes referred to by the broad phrase of monoterpene indole alkaloids.[1][2] Strictosidine is an intermediate in the biosynthesis of numerous pharmaceutically valuable metabolites including quinine, camptothecin, ajmalicine, serpentine, vinblastine, vincristine and mitragynine.
Biosynthetic pathways help to define the subgroups of strictosidine derivatives.[3][4]
Distribution
Strictosidine is found in the following plant families:
Here especially in Rhazya stricta and Catharanthus roseus.
Recent efforts in metabolic engineering have permitted the synthesis of strictosidine by yeast (Saccharomyces cerevisiae).[5] This was accomplished by adding 21 genes and 3 gene deletions.
Research
The involvement of the glucoalkaloid strictosidine in the antimicrobial and antifeedant activity of Catharanthus roseus leaves was studied. Strictosidine and its deglucosylation product, specifically formed by the enzyme strictosidine glucosidase, were found to be active against several microorganisms.[6]
References
- ↑ Mizukami, H; Nordlöv, H; Lee, S. L.; Scott, A. I. (1979). "Purification and properties of strictosidine synthetase (an enzyme condensing tryptamine and secologanin) from Catharanthus roseus cultured cells". Biochemistry 18 (17): 3760–3. doi:10.1021/bi00584a018. PMID 476085.
- ↑ Treimer, J. F.; Zenk, M. H. (1979). "Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation". European Journal of Biochemistry 101 (1): 225–33. doi:10.1111/j.1432-1033.1979.tb04235.x. PMID 510306.
- ↑ David S Seigler (1998). Plant Secondary Metabolism. Springer.
- ↑ Michael Wink (2010). Biochemistry of Plant Secondary Metabolism. Blackwell.
- ↑ Brown, S; Clastre, M; Courdavault, V; O'Connor, S. E. (2015). "De novo production of the plant-derived alkaloid strictosidine in yeast". Proceedings of the National Academy of Sciences 112 (11): 3205–3210. doi:10.1073/pnas.1423555112. PMID 25675512. Bibcode: 2015PNAS..112.3205B.
- ↑ Luijendijk, T. J. C., van der Meijden, E. & Verpoorte, R. (1996). Involvement of strictosidine as a defensive chemical inCatharanthus roseus. Journal of Chemical Ecology, 22(8), 1355–1366. https://doi.org/10.1007/bf02027718
 |

