Chemistry:Quinine
Quinine is a medication used to treat malaria and babesiosis.[1] This includes the treatment of malaria due to Plasmodium falciparum that is resistant to chloroquine when artesunate is not available.[1][2] While sometimes used for nocturnal leg cramps, quinine is not recommended for this purpose due to the risk of serious side effects.[1] It can be taken by mouth or intravenously.[1] Malaria resistance to quinine occurs in certain areas of the world.[3] Quinine is also used as an ingredient in tonic water and other beverages to impart a bitter taste.[4]
Common side effects include headache, ringing in the ears, vision issues, and sweating.[1] More severe side effects include deafness, low blood platelets, and an irregular heartbeat.[1] Use can make one more prone to sunburn.[1] While it is unclear if use during pregnancy carries potential for fetal harm, treating malaria during pregnancy with quinine when appropriate is still recommended.[1] Quinine is an alkaloid, a naturally occurring chemical compound. It possesses a C
9H
7N quinoline functional group (pyridine fused to benzene).[1]
Quinine was first isolated in 1820 from the bark of a cinchona tree, which is native to Peru,[1][5][6] and its molecular formula was determined by Adolph Strecker in 1854.[7] The class of chemical compounds to which it belongs is thus called the cinchona alkaloids. Bark extracts had been used to treat malaria since at least 1632 and it was introduced to Spain as early as 1636 by Jesuit missionaries returning from the New World.[8] It is on the World Health Organization's List of Essential Medicines.[9] Treatment of malaria with quinine marks the first known use of a chemical compound to treat an infectious disease.[10]
Uses
Medical
As of 2006, quinine is no longer recommended by the World Health Organization (WHO) as a first-line treatment for malaria, because there are other substances that are equally effective with fewer side effects. They recommend that it be used only when artemisinins are not available. [11][12][13][14] Quinine is also used to treat lupus and arthritis.
Quinine was frequently prescribed as an off-label treatment for leg cramps at night, but this has become less common since 2010 due to a warning from the US Food and Drug Administration (FDA) that such practice is associated with life-threatening side effects.[15][16][17] Quinine can also act as a competitive inhibitor of monoamine oxidase (MAO), an enzyme that removes neurotransmitters from the brain. As an MAO inhibitor, it has potential to serve as a treatment for individuals with psychological disorders, similar to antidepressants that inhibit MAO.[18]
Available forms
Quinine is a basic amine and is usually provided as a salt. Various existing preparations include the hydrochloride, dihydrochloride, sulfate, bisulfate and gluconate. In the United States, quinine sulfate is commercially available in 324 mg tablets under the brand name Qualaquin.
All quinine salts may be given orally or intravenously (IV); quinine gluconate may also be given intramuscularly (IM) or rectally (PR).[19][20] The main problem with rectal administration is that the dose can be expelled before it is completely absorbed; in practice, this is corrected by giving a further half dose. No injectable preparation of quinine is licensed in the US; quinidine is used instead.[21][22]
| Name | Amount equivalent to 100 mg quinine base |
|---|---|
| Quinine base | 100 mg |
| Quinine bisulfate | 169 mg |
| Quinine dihydrochloride | 122 mg |
| Quinine gluconate | 160 mg |
| Quinine hydrochloride | 111 mg |
| Quinine sulfate dihydrate [(quinine)2H2SO4∙2H2O] | 121 mg |
Beverages

Quinine is a flavor component of tonic water and bitter lemon soft drinks. On the soda gun behind many bars, tonic water is designated by the letter "Q" representing quinine.[23]
Tonic water was initially marketed as a means of delivering quinine to consumers in order to offer anti-malarial protection. According to tradition, because of the bitter taste of anti-malarial quinine tonic, British colonials in India mixed it with gin to make it more palatable, thus creating the gin and tonic cocktail, which is still popular today.[24] While it is possible to drink enough tonic water to temporarily achieve quinine levels that offer anti-malarial protection, it is not a sustainable long-term means of protection.[25]
In France, quinine is an ingredient of an apéritif known as quinquina, or "Cap Corse", and the wine-based apéritif Dubonnet. In Spain, quinine (also known as "Peruvian bark" for its origin from the native cinchona tree) is sometimes blended into sweet Malaga wine, which is then called "Malaga Quina". In Italy, the traditional flavoured wine Barolo Chinato is infused with quinine and local herbs, and is served as a digestif. In Britain, the company A.G. Barr uses quinine as an ingredient in the carbonated and caffeinated beverage Irn-Bru. In Uruguay and Argentina, quinine is an ingredient of a PepsiCo tonic water named Paso de los Toros. In Denmark, it is used as an ingredient in the carbonated sports drink Faxe Kondi made by Royal Unibrew.
As a flavouring agent in drinks, quinine is limited to 83 ppm (83 mg/L) in the United States,[26][27] to 85 mg/L in Taiwan,[28] and to 100 mg/L in the European Union.[29][30]
Direct use of cinchona bark in beverages is also allowed in the US, with a maximum allowed total cinchona alkaloid level of 83 ppm in the finished beverage.[31]
Scientific
Quinine (and quinidine) are used as the chiral moiety for the ligands used in Sharpless asymmetric dihydroxylation as well as for numerous other chiral catalyst backbones. Because of its relatively constant and well-known fluorescence quantum yield, quinine is used in photochemistry as a common fluorescence standard.[32][33]
Contraindications
Because of the narrow difference between its therapeutic and toxic effects, quinine is a common cause of drug-induced disorders, including thrombocytopenia and thrombotic microangiopathy.[34] Even from minor levels occurring in common beverages, quinine can have severe adverse effects involving multiple organ systems, among which are immune system effects and fever, hypotension, hemolytic anemia, acute kidney injury, liver toxicity, and blindness.[34] In people with atrial fibrillation, conduction defects, or heart block, quinine can cause heart arrhythmias, and should be avoided.[35] Quinine can cause hemolysis in G6PD deficiency (an inherited deficiency), but this risk is small and the physician should not hesitate to use quinine in people with G6PD deficiency when there is no alternative.[36]
While not necessarily an absolute contraindication, concomitant administration of quinine with drugs primarily metabolized by CYP2D6 may lead to higher than expected plasma concentrations of the drug, due to quinine's strong inhibition of the enzyme.[37]
Adverse effects
Quinine can cause unpredictable serious and life-threatening blood and cardiovascular reactions including low platelet count and hemolytic–uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP), long QT syndrome and other serious cardiac arrhythmias including torsades de pointes, blackwater fever, disseminated intravascular coagulation, leukopenia, and neutropenia.[1] Some people who have developed TTP due to quinine have gone on to develop kidney failure.[1][36] It can also cause serious hypersensitivity reactions including anaphylactic shock, urticaria, serious skin rashes, including Stevens–Johnson syndrome and toxic epidermal necrolysis, angioedema, facial edema, bronchospasm, granulomatous hepatitis, and itchiness.[1][36]
The most common adverse effects involve a group of symptoms called cinchonism, which can include headache, vasodilation and sweating, nausea, tinnitus, hearing impairment, vertigo or dizziness, blurred vision, and disturbance in color perception.[1][34][36] More severe cinchonism includes vomiting, diarrhea, abdominal pain, deafness, blindness, and disturbances in heart rhythms.[36] Cinchonism is much less common when quinine is given by mouth, but oral quinine is not well tolerated (quinine is exceedingly bitter and many people will vomit after ingesting quinine tablets).[1] Other drugs, such as Fansidar (sulfadoxine with pyrimethamine) or Malarone (proguanil with atovaquone), are often used when oral therapy is required. Quinine ethyl carbonate (quinine etabonate) is tasteless and odourless,[38] but is available commercially only in Japan. Blood glucose, electrolyte and cardiac monitoring are not necessary when quinine is given by mouth.
Quinine has diverse unwanted interactions with numerous prescription drugs, such as potentiating the anticoagulant effects of warfarin.[1] It is a strong inhibitor of CYP2D6,[37] an enzyme involved in the metabolism of many drugs.
Mechanism of action
This section is missing information about cramp mechanism; MAOI action aforementioned. (December 2022) |
Quinine is used for its toxicity to the malarial pathogen, Plasmodium falciparum, by interfering with its ability to dissolve and metabolize hemoglobin.[1][39] As with other quinoline antimalarial drugs, the precise mechanism of action of quinine has not been fully resolved, although in vitro studies indicate it inhibits nucleic acid and protein synthesis, and inhibits glycolysis in P. falciparum.[1] The most widely accepted hypothesis of its action is based on the well-studied and closely related quinoline drug, chloroquine. This model involves the inhibition of hemozoin biocrystallization in the heme detoxification pathway, which facilitates the aggregation of cytotoxic heme. Free cytotoxic heme accumulates in the parasites, causing their deaths.[40] Quinine may target the malaria purine nucleoside phosphorylase enzyme.[41]
Chemistry
The UV absorption of quinine peaks around 350 nm (in UVA). Fluorescent emission peaks at around 460 nm (bright blue/cyan hue).[42] Quinine is highly fluorescent (quantum yield ~0.58) in 0.1 M sulfuric acid solution.[32][33]
Synthesis
Cinchona trees remain the only economically practical source of quinine. However, under wartime pressure during World War II, research towards its synthetic production was undertaken. A formal chemical synthesis was accomplished in 1944 by American chemists R.B. Woodward and W.E. Doering.[43] Since then, several more efficient quinine total syntheses have been achieved,[44] but none of them can compete in economic terms with isolation of the alkaloid from natural sources. The first synthetic organic dye, mauveine, was discovered by William Henry Perkin in 1856 while he was attempting to synthesize quinine.
Biosynthesis
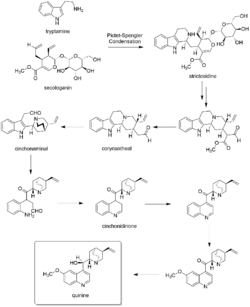
In the first step of quinine biosynthesis, the enzyme strictosidine synthase catalyzes a stereoselective Pictet–Spengler reaction between tryptamine and secologanin to yield strictosidine.[45][46] Suitable modification of strictosidine leads to an aldehyde. Hydrolysis and decarboxylation would initially remove one carbon from the iridoid portion and produce corynantheal. Then the tryptamine side-chain were cleaved adjacent to the nitrogen, and this nitrogen was then bonded to the acetaldehyde function to yield cinchonaminal. Ring opening in the indole heterocyclic ring could generate new amine and keto functions. The new quinoline heterocycle would then be formed by combining this amine with the aldehyde produced in the tryptamine side-chain cleavage, giving cinchonidinone. For the last step, hydroxylation and methylation gives quinine.[47][48]
Catalysis
Quinine and other Cinchona alkaloids can be used as catalysts for stereoselective reactions in organic synthesis.[49]: Table 3B Plate 560 For example, the quinine-catalyzed Michael addition of a malononitrile to α,β-enones gives a high degree of stereochemical control.[49]
History
Quinine was used as a muscle relaxant by the Quechua people, who are indigenous to Peru, Bolivia and Ecuador, to halt shivering.[50] The Quechua would mix the ground bark of cinchona trees with sweetened water to offset the bark's bitter taste, thus producing something similar to tonic water.[51]
Spanish Jesuit missionaries were the first to bring cinchona to Europe. The Spanish had observed the Quechua's use of cinchona and were aware of the medicinal properties of cinchona bark by the 1570s or earlier: Nicolás Monardes (1571) and Juan Fragoso (1572) both described a tree, which was subsequently identified as the cinchona tree, whose bark was used to produce a drink to treat diarrhea.[52] Quinine has been used in unextracted form by Europeans since at least the early 17th century.[53]
A popular story of how it was brought to Europe by the Countess of Chinchon was debunked by medical historian Alec Haggis around 1941.[54] During the 17th century, malaria was endemic to the swamps and marshes surrounding the city of Rome. It had caused the deaths of several popes, many cardinals and countless common Roman citizens. Most of the Catholic priests trained in Rome had seen malaria patients and were familiar with the shivering brought on by the febrile phase of the disease.
The Jesuit Agostino Salumbrino (1564–1642),[55] an apothecary by training who lived in Lima (now in present-day Peru), observed the Quechua using the bark of the cinchona tree to treat such shivering. While its effect in treating malaria (and malaria-induced shivering) was unrelated to its effect in controlling shivering from rigors, it was a successful medicine against malaria. At the first opportunity, Salumbrino sent a small quantity to Rome for testing as a malaria treatment.[56] In the years that followed, cinchona bark, known as Jesuit's bark or Peruvian bark, became one of the most valuable commodities shipped from Peru to Europe. When King Charles II was cured of malaria at the end of the 17th Century with quinine, it became popular in London.[57] It remained the antimalarial drug of choice until the 1940s, when other drugs took over.[58]
The form of quinine most effective in treating malaria was found by Charles Marie de La Condamine in 1737.[59][60] In 1820, French researchers Pierre Joseph Pelletier and Joseph Bienaimé Caventou first isolated quinine from the bark of a tree in the genus Cinchona – probably Cinchona pubescens – and subsequently named the substance.[61] The name was derived from the original Quechua (Inca) word for the cinchona tree bark, quina or quina-quina, which means "bark of bark" or "holy bark". Prior to 1820, the bark was dried, ground to a fine powder, and mixed into a liquid (commonly wine) in order to be drunk. Large-scale use of quinine as a malaria prophylaxis started around 1850. In 1853 Paul Briquet published a brief history and discussion of the literature on "quinquina".[62]
Quinine played a significant role in the colonization of Africa by Europeans. The availability of quinine for treatment had been said to be the prime reason Africa ceased to be known as the "white man's grave". A historian said, "it was quinine's efficacy that gave colonists fresh opportunities to swarm into the Gold Coast, Nigeria and other parts of west Africa".[63]
To maintain their monopoly on cinchona bark, Peru and surrounding countries began outlawing the export of cinchona seeds and saplings in the early 19th century. In 1865, Manuel Incra Mamani collected seeds from a plant particularly high in quinine and provided them to Charles Ledger. Ledger sent them to his brother, who sold them to the Dutch government. Mamani was arrested on a seed collecting trip in 1871, and beaten so severely, likely because of providing the seeds to foreigners, that he died soon afterwards.[64]
By the late 19th century the Dutch grew the plants in Indonesian plantations. Soon they became the main suppliers of the tree. In 1913 they set up the Kina Bureau, a cartel of cinchona producers charged with controlling price and production.[65] By the 1930s Dutch plantations in Java were producing 22 million pounds of cinchona bark, or 97% of the world's quinine production.[63] U.S. attempts to prosecute the Kina Bureau proved unsuccessful.[65]
During World War II, Allied powers were cut off from their supply of quinine when Germany conquered the Netherlands, and Japan controlled the Philippines and Indonesia. The US had obtained four million cinchona seeds from the Philippines and began operating cinchona plantations in Costa Rica. Additionally, they began harvesting wild cinchona bark during the Cinchona Missions. Such supplies came too late. Tens of thousands of US troops in Africa and the South Pacific died of malaria due to the lack of quinine.[63] Despite controlling the supply, the Japanese did not make effective use of quinine, and thousands of Japanese troops in the southwest Pacific died as a result.[66][67][68][69]
Quinine remained the antimalarial drug of choice until after World War II. Since then, other drugs that have fewer side effects, such as chloroquine, have largely replaced it.[70]
Bromo Quinine were brand name cold tablets containing quinine, manufactured by Grove Laboratories. They were first marketed in 1889 and available until at least the 1960s.[71]
Conducting research in central Missouri, John S. Sappington independently developed an anti-malaria pill from quinine. Sappington began importing cinchona bark from Peru in 1820. In 1832, using quinine derived from the cinchona bark, Sappington developed a pill to treat a variety of fevers, such as scarlet fever, yellow fever, and influenza in addition to malaria. These illnesses were widespread in the Missouri and Mississippi valleys. He manufactured and sold "Dr. Sappington's Anti-Fever Pills" across Missouri. Demand became so great that within three years, Sappington founded a company known as Sappington and Sons to sell his pills nationwide.[72]
Society and culture
Natural occurrence
The bark of Remijia contains 0.5–2% of quinine. The bark is cheaper than bark of Cinchona. As it has an intense taste, it is used for making tonic water.[73]
Regulation in the US
From 1969 to 1992, the US Food and Drug Administration (FDA) received 157 reports of health problems related to quinine use, including 23 which had resulted in death.[74] In 1994, the FDA banned the marketing of over-the-counter quinine as a treatment for nocturnal leg cramps. Pfizer Pharmaceuticals had been selling the brand name Legatrin for this purpose. It is also sold as a softgel (by SmithKlineBeecham) as Q-vel. Doctors may still prescribe quinine, but the FDA has ordered firms to stop marketing unapproved drug products containing quinine. The FDA is also cautioning consumers about off-label use of quinine to treat leg cramps.[15][16] Quinine is approved for treatment of malaria, but was also commonly prescribed to treat leg cramps and similar conditions. Because malaria is life-threatening, the risks associated with quinine use are considered acceptable when used to treat that condition.[75]
Though Legatrin was banned by the FDA for the treatment of leg cramps, the drug manufacturer URL Mutual has branded a quinine-containing drug named Qualaquin. It is marketed as a treatment for malaria and is sold in the United States only by prescription. In 2004, the CDC reported only 1,347 confirmed cases of malaria in the United States.[76]
Termination of pregnancy
For much of the 20th century, women's use of an overdose of quinine to deliberately terminate a pregnancy was a relatively common abortion method in various parts of the world, including China.[77]
Cutting agent
Quinine is sometimes detected as a cutting agent in street drugs such as cocaine and heroin.[78]
Other animals
Quinine is used as a treatment for Cryptocaryon irritans (commonly referred to as white spot, crypto or marine ich) infection of marine aquarium fish.[79]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 "Quinine sulfate". Drugs.com. 20 February 2020. https://www.drugs.com/monograph/quinine-sulfate.html.
- ↑ "Artemether for severe malaria". The Cochrane Database of Systematic Reviews 6 (6). June 2019. doi:10.1002/14651858.CD010678.pub3. PMID 31210357.
- ↑ "Quinoline antimalarials: mechanisms of action and resistance". International Journal for Parasitology 27 (2): 231–240. February 1997. doi:10.1016/s0020-7519(96)00152-x. PMID 9088993.
- ↑ Chemistry: The Molecular Science. Jones & Bartlett Learning. 1997. p. 137. ISBN 978-0-815-18450-8. https://books.google.com/books?id=1vnk6J8knKkC&pg=PA137.
- ↑ Traditional Medicinal Plants and Malaria. CRC Press. 28 June 2004. p. 231. ISBN 978-0-203-50232-7. https://books.google.com/books?id=L3lZiwsCZoYC&pg=PA23.
- ↑ Plant bioactives and drug discovery: principles, practice, and perspectives. Hoboken, N.J.: John Wiley & Sons. 2012. p. 2. ISBN 978-0-470-58226-8. https://books.google.com/books?id=hhraBwhymOUC&pg=PA2.
- ↑ "Ueber einen neuen aus Aldehyd - Ammoniak und Blausäure entstehenden Körper". Liebigs Ann. Chem. 91 (3): 349–351. 1854. doi:10.1002/jlac.18540910309. https://zenodo.org/record/1427060.
- ↑ Treatment and Prevention of Malaria: Antimalarial Drug Chemistry, Action and Use.. [S.l.]: Springer Verlag. 2011. p. 45. ISBN 978-3-0346-0479-6. https://books.google.com/books?id=cNuY6tyyyrUC&pg=PA45.
- ↑ The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ↑ "Quinine". https://www.britannica.com/science/quinine.
- ↑ World Health Organization (2006). "Guidelines for the treatment of malaria". World Health Organization. http://apps.who.int/malaria/docs/TreatmentGuidelines2006.pdf.
- ↑ "Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial". Lancet 366 (9487): 717–725. 2005. doi:10.1016/S0140-6736(05)67176-0. PMID 16125588.
- ↑ "Oral quinine for the treatment of uncomplicated malaria". BMJ 339. July 2009. doi:10.1136/bmj.b2066. PMID 19622550. https://researchonline.lshtm.ac.uk/id/eprint/5019/1/Oral%20quinine%20for%20the%20treatment%20of%20uncomplicated%20malaria%20_%20The%20BMJ.pdf.
- ↑ "Effectiveness of quinine versus artemether-lumefantrine for treating uncomplicated falciparum malaria in Ugandan children: randomised trial". BMJ 339. July 2009. doi:10.1136/bmj.b2763. PMID 19622553.
- ↑ 15.0 15.1 "FDA Drug Safety Communication: New risk management plan and patient Medication Guide for Qualaquin (quinine sulfate)". U.S. Food and Drug Administration (FDA). 7 August 2010. https://www.fda.gov/drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm218202.htm.
- ↑ 16.0 16.1 "Serious risks associated with using Quinine to prevent or treat nocturnal leg cramps (September 2012)". 31 August 2012. https://www.fda.gov/ForHealthProfessionals/LearningActivities/ucm317811.htm.
- ↑ "Quinine for Night-Time Leg Cramps". https://www.consumerreports.org/cro/2012/04/quinine-for-night-time-leg-cramps-no-longer-recommended/index.htm.
- ↑ "Monoamine oxidase inhibitors from Cinchonae Cortex". Chemical & Pharmaceutical Bulletin 37 (2): 363–366. February 1989. doi:10.1248/cpb.37.363. PMID 2743481.
- ↑ "Efficacy and pharmacokinetics of a new intrarectal quinine formulation in children with Plasmodium falciparum malaria". British Journal of Clinical Pharmacology 41 (5): 389–395. May 1996. doi:10.1046/j.1365-2125.1996.03246.x. PMID 8735679.
- ↑ "Safety and efficacy of rectal compared with intramuscular quinine for the early treatment of moderately severe malaria in children: randomised clinical trial". BMJ 332 (7549): 1055–1059. May 2006. doi:10.1136/bmj.332.7549.1055. PMID 16675812.
- ↑ "Treatment with quinidine gluconate of persons with severe Plasmodium falciparum infection: discontinuation of parenteral quinine from CDC Drug Service". MMWR. Recommendations and Reports 40 (RR-4): 21–23. April 1991. PMID 1850497. https://www.cdc.gov/mmwr/preview/mmwrhtml/00043932.htm.
- ↑ "Making antimalarial agents available in the United States". The New England Journal of Medicine 353 (4): 335–337. July 2005. doi:10.1056/NEJMp058167. PMID 16000347.
- ↑ Miss Charming's Guide for Hip Bartenders and Wayout Wannabes. USA: Sourcebooks, Inc.. 2006. p. 189. ISBN 978-1-4022-0804-1.
- ↑ "Gin and Tonic: The fascinating story behind the invention of the classic English cocktail". 17 March 2017. https://www.india.com/lifestyle/gin-and-tonic-the-fascinating-story-behind-the-invention-of-the-classic-english-cocktail-1934782/.
- ↑ "Editorial: Gin tonic revisited". Tropical Medicine & International Health 9 (12): 1239–1240. December 2004. doi:10.1111/j.1365-3156.2004.01357.x. PMID 15598254.
- ↑ "Effects of quinine, quinidine, and chloroquine on alpha9alpha10 nicotinic cholinergic receptors". Molecular Pharmacology 68 (3): 822–829. September 2005. doi:10.1124/mol.105.014431. PMID 15955868.
- ↑ "21 CFR 172.575 -- Quinine." (in en). https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-F/section-172.575.
- ↑ "食品添加物使用範圍及限量暨規格標準". 2020-04-14. p. 104. https://rc.csmu.edu.tw/var/file/18/1018/img/1581/339191880.pdf.
- ↑ "Scientific Opinion on Flavouring Group Evaluation 35, Revision 1 (FGE.35Rev1): Three quinine salts from the Priority list from chemical group 30". EFSA Journal 13 (9). September 2015. doi:10.2903/j.efsa.2015.4245.
- ↑ "COMMISSION IMPLEMENTING REGULATION (EU) No 872/2012". Official Journal of the European Union. http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1507509835974&uri=CELEX:32012R0872.
- ↑ "21 CFR 172.510 -- Natural flavoring substances and natural substances used in conjunction with flavors." (in en). https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-F/section-172.510. "Cinchona, red, bark"
- ↑ 32.0 32.1 "2. Instrumentation for Fluorescence Spectroscopy". Principles of Fluorescence Spectroscopy (3rd ed.). Springer Science & Business Media. 2006. p. 54. ISBN 978-0-387-46312-4. https://books.google.com/books?id=-PSybuLNxcAC.
- ↑ 33.0 33.1 "Quinine sulfate". https://omlc.org/spectra/PhotochemCAD/html/081.html.
- ↑ 34.0 34.1 34.2 "Diversity and severity of adverse reactions to quinine: A systematic review". American Journal of Hematology 91 (5): 461–466. May 2016. doi:10.1002/ajh.24314. PMID 26822544.
- ↑ "Off-label use of sildenafil in valvular heart disease should be avoided". Clinical Pharmacist. 2017. doi:10.1211/cp.2017.20203778. ISSN 2053-6178.
- ↑ 36.0 36.1 36.2 36.3 36.4 "US label: quinine sulfate". FDA. April 2013. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021799s023lbl.pdf.
- ↑ 37.0 37.1 "Pathway-specific inhibition of primaquine metabolism by chloroquine/quinine". Malaria Journal 15 (1). September 2016. doi:10.1186/s12936-016-1509-x. PMID 27618912.
- ↑ "Relative bioavailability of the hydrochloride, sulphate and ethyl carbonate salts of quinine". British Journal of Clinical Pharmacology 25 (2): 261–263. February 1988. doi:10.1111/j.1365-2125.1988.tb03299.x. PMID 3358888.
- ↑ Template:Cite DrugBank
- ↑ "Quinoline antimalarials: mechanisms of action and resistance". International Journal for Parasitology 27 (2): 231–240. February 1997. doi:10.1016/s0020-7519(96)00152-x. PMID 9088993.
- ↑ "Quinine's Target". Science. 22 January 2019. https://www.science.org/content/blog-post/quinine-s-target. Retrieved 28 January 2019.
- ↑ "Basic Concepts in Fluorescence". http://www.olympusmicro.com/primer/techniques/fluorescence/fluorescenceintro.html.
- ↑ "The Total Synthesis of Quinine". J Am Chem Soc 66 (849): 849. 1944. doi:10.1021/ja01233a516. Bibcode: 1944JAChS..66Q.849W.
- ↑ "Die Jagd auf Chinin: Etappenerfolge und Gesamtsiege" (in de). Angewandte Chemie International Edition 117 (6): 876–907. 2005. doi:10.1002/ange.200400663. Bibcode: 2005AngCh.117..876K.
- ↑ "Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation". European Journal of Biochemistry 101 (1): 225–233. November 1979. doi:10.1111/j.1432-1033.1979.tb04235.x. PMID 510306.
- ↑ "Purification and properties of strictosidine synthetase (an enzyme condensing tryptamine and secologanin) from Catharanthus roseus cultured cells". Biochemistry 18 (17): 3760–3763. August 1979. doi:10.1021/bi00584a018. PMID 476085.
- ↑ Medicinal natural products: a biosynthetic approach (3rdition ed.). Wiley. pp. 380–381. ISBN 978-0-470-74276-1.
- ↑ "Chemistry and biology of monoterpene indole alkaloid biosynthesis". Natural Product Reports 23 (4): 532–547. August 2006. doi:10.1039/b512615k. PMID 16874388.
- ↑ 49.0 49.1 "The Catalytic, Enantioselective Michael Reaction", Organic Reactions, Hoboken, New Jersey, US: John Wiley & Sons, Inc., 2016-09-13, pp. 1–898, doi:10.1002/0471264180.or090.01, ISBN 978-0-471-26418-7
- ↑ "Cortex Cinchonæ". Pharmacographia: A History of the Principal Drugs of Vegetable Origin, Met with in Great Britain and British India. London: Macmillan and Co.. 1874. pp. 302–331. https://books.google.com/books?id=ATQbAAAAYAAJ&pg=PA302.
- ↑ The Story of Trees: and how they changed the way we live. illustrated by Thibaud Hérem. London: Laurence King. 2020. p. 148. ISBN 978-1-7862-7522-6.
- ↑ See:
- "Fragoso, Monardes and pre-Chinchonian knowledge of Cinchona". Archives of Natural History 22 (2): 169–181. 1995. doi:10.3366/anh.1995.22.2.169. ISSN 0260-9541. https://www.reumatologiaclinica.org/en-pdf-S2173574307702460.
- Dangerous Garden: The Quest for Plants to Change Our Lives. Cambridge, MA: Harvard University Press. 2004. p. 28. ISBN 978-0-674-01104-5. https://books.google.com/books?id=Ze0n0yeqsXUC&pg=PA28.
- (in es) Primera y segunda y tercera partes de la Historia medicinal, de las cosas que se traen de nuestras Indias Occidentales, que sirven en Medicina. Seville, Spain: Fernando Díaz. 1580. pp. 74–75. https://books.google.com/books?id=BMiaWiCqFCMC&pg=RA1-PA75-IA2. "Del nuevo Reyno, traen una corteza, que dizen ser de un arbol, que es de mucha grandeza: el qual dizen que lleva unas hojas en forma de coraçon, y que no lleva fruto. Este arbol tiene una corteza gruessa, muy solida y dura, que en esto y en el color parece mucho a la corteza del palo que llaman Guayacan: en la superficie tiene una pelicula delgada blanquisca, quebrada por toda ella: tiene la corteza mas de un dedo de gruesso, solida y pesada: la qual gustada tiene notable amargor, como el de la Genciana: tiene en el gusto notable astriction, con alguna aromaticidad, porque al fin de mascar la respica della buen olor. Tienen los Indios esta corteza en mucho, y usan della en todo genero de camaras, que sean con sangre, o sin ella. Los Españoles fatigados de aquesta enfermedad, por aviso de los Indios, han usado de aquesta corteza y han sanado muchos dellos con ella.
Toman della tanto como una haba pequeña hecha polvos, tomase en vino tinto, o en agua apropiada, como tienen la calentura, o mal: hase de tomar por la mañana en ayunas, tres o quatro vezes: usando en lo demas, la orden y regimiento que conviene a los que tienen camaras." - (in es) Discursos de las cosas aromaticas, arboles y frutales, y de otras muchas medicinas simples que se traen de la India Oriental y que sirven al uso de medicina. Madrid, Spain: Francisco Sánchez. 1572. p. 35. https://books.google.com/books?id=ZGpIEb3BRfAC. "En el nuevo mundo ay un grande arbol que lleva las hojas a forma de coraçon, y carece de fruto. Tiene dos cortezas, la una gruessa muy solida y dura, que assi en la sustancia como en el color es muy semejante al Guayacan: la otra es mas delgada y blanquezina, la qual es amarga con alguna estipticidad: y demas desto es aromatica. Tienenla en mucho nuestros Indios, porque la usan contra qualesquier camaras, tomando del polvo peso de una drama o poco mas, desatado en agua azerada, o vino tinto."
- ↑ "Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria". Malaria Journal 10. May 2011. doi:10.1186/1475-2875-10-144. PMID 21609473.
- ↑ "The Countess and the cure". New Scientist. 15 September 2001. https://www.newscientist.com/article/mg17123085-200-the-countess-and-the-cure/.
- ↑ "Vida del Devoto Hermano Agustin Salumbrino" (in es). Varones ilustres en santidad, letras y zelo de las almas de la Compañía de Jesús. Varones ilustres de la Compañía de Jesús. 5. Original series by Juan Eusebio Nieremberg. Madrid, Spain: José Fernandez de Buendía. 3 August 1642 (published 1666). pp. 612–628. https://books.google.com/books?id=paaIyIGye1gC&pg=PA612. "Naciò el Hermano Agustin Salumbrino el año de mil y quinientos y sesenta y quatro en la Ciudad de Fḷọṛi en la Romania [...]"
- ↑ See:
- "Precisions on the History of Quinine". Reumatología Clínica. Letters to the Editor 3 (4): 194–196. 2007. doi:10.1016/S2173-5743(07)70246-0. ISSN 2173-5743. https://www.reumatologiaclinica.org/en-pdf-S2173574307702460. "In fact, though the last wordon this has not yet been spoken, there are Jesuit texts thatmention that quinine reached Rome in 1632, with theprovincial of the Jesuit missions in Peru, father AlonsoMessia Venegas, as its introducer, when he brought asample of the bark to present it as a primacy, and whohad left Lima 2 years earlier, because evidence of his stayin Seville 1632 has been registered, publishing one of hisbooks there and following his way to Rome as a procurator.".
- "El P. Diego de Torres Vazquez" (in es). Los antiguos jesuitas del Perú. Lima, Peru: Imprenta Liberal. June 1882. pp. 180–181. https://archive.org/details/losantiguosjesui00torr/page/180/mode/2up. "Al siguiente año se dirigieron á Europa los Procuradores P. Alonso Messía Venegas y P. Hernando de Leon Garavito, llevando gran cantidad de la corteza de la quina, cuyo conocimiento extendieron por el mundo los jesuitas."
- "Capítulo 10: La Condesa de Chinchón". http://lamalariayelarboldequina.blogspot.com/2013/11/capitulo-9-proximamente.html. "A últimas horas de la tarde del treinta y uno de mayo de 1631 se hizo a la vela la Armada Real con dirección a Panamá llevando el precioso cargamento de oro y plata.
En una de las naves viajaban los procuradores jesuitas padres Alonso Messia y Hernando León Garavito custodiando los fardos con la corteza de quina en polvo preparados por Salumbrino. Después de casi veinte días de navegación el inapreciable medicamento llegó a la ciudad de Panamá, donde fue descargado para cruzar en mulas el agreste camino del itsmo palúdico hasta Portobelo para seguir a Cartagena y la Habana, cruzar el Atlántico y llegar a Sanlúcar de Barrameda en Sevilla. [...] Finalmente siguió su camino a Roma y a su destino final el Hospital del Espíritu Santo."
- ↑ Quinine: malaria and the quest for a cure that changed the world. New York, NY: Perennial. 2004.
- ↑ Quinine and Quarantine. Columbia, Missouri: University of Missouri Press. 2000.
- ↑ "Sur l'arbre du quinquina". Histoire de l'Académie royale des sciences. Imprimerie Royale. 29 May 1737 (published 1740). pp. 226–243. https://books.google.com/books?id=yOAEAAAAQAAJ&pg=PA226.
- ↑ De Jussieu accompanied de la Condamine on the latter's expedition to Peru: Description de l'arbre à quinquina. Paris: Société du traitement des quinquinas. 1737 (published 1934). https://gallica.bnf.fr/view3if/ga/ark:/12148/bpt6k90339p.
- ↑ "Recherches Chimiques sur les Quinquinas" (in fr). Annales de Chimie et de Physique (Crochard) 15: 337–365. 1820. https://books.google.com/books?id=veE3AAAAMAAJ&pg=PA337. "The authors name quinine on page 348: " ..., nous avons cru devoir la nommer quinine, pour la distinguer de la cinchonine par un nom qui indique également son origine."".
- ↑ (in fr) Traité thérapeutique du quinone et de ses préparations. Paris: L. Martinet. 1853. https://archive.org/details/b23982135.
- ↑ 63.0 63.1 63.2 A People's History of Science: Miners, Midwives, and 'Low Mechanicks'. New York: Nation Books. 2005. pp. 95–96. ISBN 978-1-56025-748-6. https://archive.org/details/peopleshistoryof0000conn. Also cites The Greatest Benefit to Mankind: A Medical History of Humanity. New York: W. W. Norton. 1998. pp. 465–466. ISBN 978-0-393-04634-2. https://archive.org/details/greatestbenefitt00port/page/465.
- ↑ "Hunting lost plants in botanical collections" (in en). 7 April 2022. https://wellcomecollection.org/articles/YjyPpREAAB8AhS-R.
- ↑ 65.0 65.1 The Fever: How Malaria Has Ruled Humankind for 500,000 Years. Farrar, Straus and Giroux. 2010. p. 94.
- ↑ "29". The Fall of the Philippines. Washington, D.C.: United States Army. 1953. p. 524. http://www.history.army.mil/books/wwii/5-2/5-2_29.htm.
- ↑ "Remembering the war in New Guinea: Japanese Medical Corps – malaria". http://ajrp.awm.gov.au/AJRP/remember.nsf/Web-Printer/1989A520D772FE7ECA256B5A0011AF2B?OpenDocument.
- ↑ "8". Preventive Medicine in World War II: Volume VI, Communicable Diseases: Malaria. Washington, D.C.: Department of the Army. 1963. pp. 401 and 434. http://history.amedd.army.mil/booksdocs/wwii/Malaria/chapterVIII.htm.
- ↑ "Notes on Japanese Medical Services". Tactical and Technical Trends (36). 1943. http://www.lonesentry.com/articles/ttt/japanese_medical_services.html.
- ↑ The Fever: How Malaria Has Ruled Humankind for 500,000 Years. Farrar, Straus and Giroux. 2010. p. 102.
- ↑ "Medicine: What's Good for a Cold?". Time. 22 February 1960. http://www.time.com/time/magazine/article/0,9171,939616,00.html.
- ↑ "John. S Sappington". Historic Missourians. State Historical Society of Missouri. https://historicmissourians.shsmo.org/historicmissourians/name/s/sappington/.
- ↑ (in cs) Šest rostlin, které změnily svět. Prague: Akademie věd České republiky. 2004. p. 59. ISBN 978-80-200-1179-4.
- ↑ "FDA Orders Stop to Marketing of Quinine for Night Leg Cramps". FDA Consumer Magazine. U.S. Food and Drug Administration (FDA). July–August 1995. https://www.fda.gov/fdac/departs/695_updates.html.
- ↑ "FDA Orders Unapproved Quinine Drugs from the Market and Cautions Consumers About Off-Label Use of Quinine to Treat Leg Cramps" (Press release). U.S. Food and Drug Administration (FDA). 11 December 2006. Archived from the original on 28 July 2009. Retrieved 31 July 2009.
- ↑ "Malaria surveillance--United States, 2004". Morbidity and Mortality Weekly Report. Surveillance Summaries 55 (4): 23–37. May 2006. PMID 16723971. https://www.cdc.gov/mmwr/PDF/ss/ss5301.pdf.
- ↑ Reproductive realities in modern China: birth control and abortion, 1911-2021. Cambridge, United Kingdom: Cambridge University Press. 2023. p. 1. ISBN 978-1-009-02733-5. OCLC 1366057905.
- ↑ "Dimethyltryptamine and Ecstasy Mimic Tablets (Actually Containing 5-Methoxy-Methylisopropyltryptamine) in Oregon". Drug Enforcement Administration, U.S. Department of Justice. October 2009. p. 79. https://www.justice.gov/dea/pr/micrograms/2009/mg1009.pdf.
- ↑ "Cryptocaryon irritans". Reef Culture Magazine. http://www.reefculturemagazine.com.au/cryptocaryon.html. Retrieved 9 July 2009.
Further reading
- The encyclopedia of Civil War medicine. Armonk, NY: Sharpe, Inc.. 2008.
- Seeds of Change: Six Plants That Transformed Mankind. Berkeley, CA: Counterpoint. 2005. ISBN 978-1-59376-049-6.
- "Aeromedical considerations of malaria prophylaxis with mefloquine hydrochloride". Aviation, Space, and Environmental Medicine 53 (10): 1011–1013. October 1982. PMID 6983345.
- "Ocular quinine toxicity treated with hyperbaric oxygen". Undersea & Hyperbaric Medicine 24 (2): 131–134. June 1997. PMID 9171472. http://archive.rubicon-foundation.org/2278. Retrieved 13 August 2008.
- War and disease: biomedical research on malaria in the twentieth century. New Brunswick, NJ: Rutgers University Press. 2009.
- "Lords of Industry". The North American Review (University of Northern Iowa) 138 (331): 535–553. June 1884. ISSN 0029-2397.
- Guidelines for the treatment of malaria (3rd ed.). World Health Organization (WHO). 2015. ISBN 978-92-4-154912-7.
External links
- Quinine at the Drug Information Portal - U.S. National Library of Medicine
- Quinine at the International Programme on Chemical Safety
- "Quinine". Resource Center. Chemwatch. https://www.chemwatch.net/resource-center/quinine/.
 |
