Chemistry:Cerium(III) methanesulfonate
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
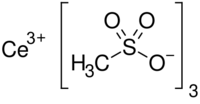
| Ce(CH3SO3)3 | |
| Molar mass | 461.46 g/mol |
| Appearance | White crystalline solid[1] |
| Insoluble in water | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cerium(III) methanesulfonate is a white salt, usually found as the dihydrate with the formula Ce(CH3SO3)3·2H2O that precipitates from the neutralisation of cerium(III) carbonate with methanesulfonic acid, as first reported by L.B. Zinner in 1979.[2][3] The crystals have a monoclinic polymeric structure were each methanesulfonate ion forms bonds with two cerium atoms, which present a coordination number of 8.[4] The anhydrous salt is formed by water loss at 120 °C. Similar methanesulfonates can be prepared with other lanthanides.[5] Cerium(III) methanesulfonate in solution is used as a precursor of electrogenerated cerium(IV), which is a strong oxidant and whose salts can be used in organic synthesis.[6] The same principle of Ce(IV) electrogeneration is the fundamental reaction in the positive half-cell of the zinc–cerium battery.
See also
References
- ↑ Kreh, Robert P. (2001). "Cerium (III) Methanesulfonate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc044. ISBN 0471936235.
- ↑ Zinner, L.B. (1979). "Hydrated lanthanide methanesulfonates". Anais da Academia Brasileira de Ciências 30: 27.
- ↑ Zinner, L.B. (1980). "Anhydrous lanthanide (III) methanesulfonates". Anais da Academia Brasileira de Ciências 52 (4): 715.
- ↑ Aricó, E.M; Zinner, L.B.; Apostolidis, C.; Dornberger, E.; Kanellakopulos, B.; Rebizant, J. (1997). "Structures of the anhydrous Yb(III) and the hydrated Ce(III), Sm(III) and Tb(III) methanesulfonates". Journal of Alloys and Compounds 249 (1–2): 111–115. doi:10.1016/s0925-8388(96)02756-9.
- ↑ Aricó, E.M.; Zinner, L.B.; Kanellakopulos, B.; Dornberger, E.; Rebizante, J.; Apostolidis, C. (2001). "Structure and properties of hydrated La(III), Nd(III) and Er(III) methanesulfonates". Journal of Alloys and Compounds 323–324: 39–44. doi:10.1016/s0925-8388(01)00975-6.
- ↑ Kreh, Robert P. (1989). "Mediated electrochemical synthesis of aromatic aldehydes, ketones, and quinones using ceric methanesulfonate". The Journal of Organic Chemistry 54 (7): 1526–1531. doi:10.1021/jo00268a010.
 |

