Chemistry:Cerium nitrate

| |
| Identifiers | |
|---|---|
| |
PubChem CID
|
|
| UNII |
|
| Properties | |
| Ce(NO3)3 | |
| Molar mass | 326.12 g/mol |
| Appearance | Colorless crystals (hexahydrate) |
| Density | 2.38 g cm−3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cerium nitrate refers to a family of nitrates of cerium in the three or four oxidation state. Often these compounds contain water, hydroxide, or hydronium ions in addition to cerium and nitrate. Double nitrates of cerium also exist.
Cerium(III) nitrates
Anhydrous cerous nitrate, also called cerium(III) nitrate, is the anhydrous salt with the formula Ce(NO3)3.(CAS number 10108-73-3).
Cerium nitrate hexahydrate, with the formula Ce(NO3)3.6H2O (CAS number 10294-41-4) is the most common nitrate of cerium(III). It is a component in a burn treatment cream that also includes silver sulphadiazine. Concentrations used are 0.5 M for the cerium nitrate. For very serious burns it reduces the death rate.[1][2] At 150 °C the hexahydrate loses water of crystallization to make a trihydrate, which itself decomposes above 200 °C.[3] Cerous nitrate hexahydrate has pinacoidal triclinic crystals.[4]
Hydronium cerium(III) nitrate hydrate, Ce(NO3)5(H3O)2.H2O[5] It is monoclinic with space group P2/c.[5] The diaquapentanitratocerate(III) anion (Ce(NO3)5(H2O)2)2− occurs in several salts. The salts have extreme non-linear optical properties.[6]
Cerium(IV) nitrates
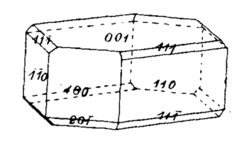
Cerium tetranitrate pentahydrate is prepared by evaporating a solution of ceric nitrate in concentrated nitric acid. It forms orthorhombic crystals with bipyramidal shape. The common crystal face Miller index is {111}, But it can have smaller faces with Miller index {010} and {110}. The density is 2.403 g/cm3. Its optical properties are that it is biaxial with 2V of 34°, and strongly dispersive. On its B and C axes it appears yellow, but orange red on the A axis.[7]
Ceric nitrate is quite soluble in non polar solvents such as ethyl ether. Ether will extract the cerium nitrate from 5N nitric acid.[8] In nitric acid, nitrato ceric acid[9] (H2[Ce(NO3)6] and H[Ce(NO3)5.H2O]) are present. The solubility of this nitrate in non-polar solvents allows the separation of cerium from other rare earths.[8]
Basic cerium(IV) nitrate has the formula Ce(NO3)3.OH.3H2O. It also forms upon evaporation of solutions of cerium(IV) in nitric acid.[10] When this meets ammonia in water solution it reacts to form ceric ammonium nitrate and ceric hydroxide.[10]
Basic dicerium nitrate has the formula Ce2O(NO3)6(H2O)6·2H2O. Again it crystallizes from solutions of cerium(IV) in nitric acid. It crystallises as monoclinic crystals with space group P21lc with unit cell dimensions a=8.723 Å b=8.940 Å c=13.981 Å, β = 94.91°. Each unit cell contains two formula units Ce2O(NO3)6(H2O)3 and Ce2O(NO3)6 form when this basic nitrate is heated slowly to 180 °C in a vacuum.[11]
Ammonium and alkali metal cerium nitrates
The diaquapentanitratocerate(III) anion (Ce(NO3)5(H2O)2)2− occurs in several salts. The salts have extreme non-linear optical properties.[6]
K2Ce(NO3)5[12] crystals can be grown by evaporating a solution of potassium nitrate, cerous nitrate, and nitric acid. Each cerium atom is surrounded by the oxygen atoms of five bidentate nitrate groups and two water oxygen atoms.[6] It can be grown into optical quality crystals of around 100 cm3 in 12 weeks.[6] Crystals are colourless.[6] The space group of the crystal is Fdd2 and their form is orthorhombic.[6] Potassium cerium nitrate was probably discovered by L. Th. Lange in 1861.[13] However it was only properly described in 1894 by Fock.[14][15] Even then the amount of water in the substance was wrong and it took till 1911 when Jantsch & Wigdorow correctly stated that there were two water molecules.[16] The non-linear optical effects were found in 1993. For optical applications it is known as KCN.[17]
Diammonium diaquapentanitratocerate dihydrate.[16][18] Its Raman spectrum has been published.[19] It is quite soluble in water with 100 ml dissolving 235 grams at 9 °C and 817 grams at 65°.[20]
- dirubidium diaquapentanitratocerate dihydrate.[16]
- dicaesium diaquapentanitratocerate dihydrate, or caesium cerous nitrate Cs2Ce(NO3)5.2H2O forms monoclinic crystals with crystal parameters a/b=1.2052, c/b=0.9816 and β = 103°41'.[20]
- dithallium diaquapentanitratocerate dihydrate.[16]
- Bis{4-[(4H-1,2,4-triazol-4-yl)iminomethyl]pyridinium} diaquapentanitratocerate. (C8H8N5)2[Ce(NO3)5(H2O)2] is monoclinic with space group C2/c.[21]
| name | formula | melt | density | a | b | c | β° | Vol | Z |
|---|---|---|---|---|---|---|---|---|---|
| dipotassium diaquapentanitratocerate | K2Ce(NO3)5·2 H2O | 2.543 | 11.263 | 21.404 | 12.230 | 90 | 2948 | 8[6] | |
| dipotassium hexanitratocerate[22] | K2Ce(NO3)6 | ||||||||
| tripotassium dicerium(III) nitrate[22] | K3Ce2(NO3)9 | 2.525 | 13.597 | 13.597 | 13.597 | 90 | 2514 | 4 | |
| diammonium diaquapentanitratocerate dihydrate | (NH4)2Ce(NO3)5·4 H2O | 2.128 | 11.09 | 8.936 | 17.96 | 101.77 | 1743[23][18] | 4 | |
| dirubidium diaquapentanitratocerate dihydrate | Rb2Ce(NO3)5·4 H2O | 70° | 2.497 | 11.050 | 8.977 | 17.859 | 100.88[24] | ||
| dicaesium diaquapentanitratocerate dihydrate ? | Cs2Ce(NO3)5·4 H2O | ||||||||
| dithallium diaquapentanitratocerate dihydrate ? | Tl2Ce(NO3)5·4 H2O | 64.5° | 3.326 | ||||||
| Bis{4-[(4H-1,2,4-triazol-4-yl)iminomethyl]pyridinium} diaquapentanitratocerate | (C8H8N5)2[Ce(NO3)5(H2O)2] | 10.322 | 16.126 | 17.575 | 100.107 | 2883.2 | 4 | ||
| 1,10-Phenanthroline-H diaquapentanitratocerate | HPhen2[Ce(NO3)5(H2O)2] | 1.83 | 7.5534 | 8.083 | 25.8377 | 89.947 β=89.937 γ=86.981 | 1572.94 | 2[25] | |
| Hydronium Cerium (III) Nitrate Hydrate | Ce(NO3)5(H3O)2·H2O | 21.36 | 7.899 | 15.133 | 91.02 | 8 |
Divalent double nitrates
Cerous magnesium nitrate is the first discovered member of a divalent series CeM(II)(NO3)5. This has an extremely low Kapitza resistance to liquid 3He. At the time of discovery it value was only 1% of the previous record holder. Low thermal resistance is important at temperatures below 1K, because there is not much temperature difference to cause a large heat flow rate, and cooling can take an excessive time if there are barriers to heat transfer.[26] [27]
Other cerous double nitrates
Cerous sodium nitrate monohydrate, Na2Ce(NO3)5.H2O has density 2.641 g/cm3. It can be made by boiling the stoichiometric mixture of cerous nitrate, and sodium nitrate in nitric acid, and then evaporating at 40 °C. The crystals are clear rod shaped monoclinic with space group P2/c. Crystal cell sizes are a=21.387 b=7.9328 c=15.184 β=90.657 V=2576 formulas per cell Z=8. The way the components are arranged in the crystal is that there are six nitrates around each cerium atom, however to get to the average of five per cerium, two nitrate groups on each, link the atoms into a chain along the an axis.[28]
There are anhydrous double nitrates such as Ce2Rb3(NO3)9 and Ce2K3(NO3)9.[29] The potassium salt, Ce2K3(NO3)9 can be made by using the water solution of potassium nitrate and cerous nitrate in 3:2 molar ratio, evaporated at 40 °C. The crystals are colourless cubic from space group P4132. Its formula weight is 955.6. Three formulas exist in each unit cell which at 20 °C, has a volume of 2514.1 Å3 and cell side of a=13.597 Å. The density is 2.525 g/cm. In this compound each cerium atoms is surrounded by twelve oxygen atoms from six nitrate groups. Three of the nitrates form a bridge in each of three dimensions. These bridges form three spirals each at 90° to each other along the crystal axes.[22]
A related series with ratio 1.5 of the monovalent ion to cerium includes 2Ce(NO3)3.3(NH4)NO3.12H2O[20]
A mixed caesium, sodium cerium triple nitrate Cs2NaCe(NO3)6 crystallizes in the cubic system. The unit cell size is 1.1196 nm with volume of 1.4034 nm3 and four molecules per cell.[30]
Ceric double nitrates
The alkali metals form orange-red monoclinic crystals as a double salt with ceric nitrate: M2[Ce(NO3)6] with M=K, Rb, Cs, or [NH4].[10]
- Ceric ammonium nitrate contains the icosahedral shaped ion [Ce(NO3)6]2− which has cerium in the +4 oxidation state.[31] It is used as a reagent in oxidimetry.[9]
- Ceric potassium nitrate K2[Ce(NO3)6] has two different crystal forms, hexagonal and monoclinic. Slow evaporation and crystallization results in the monoclinic form. But fast crystallization results in a mixture of the two shapes. Both of these forms have six nitrate groups connected via two oxygens each to the cerium [Ce(NO3)6]2−. The substance is made by dissolving ceric hydroxide in nitric acid with the appropriate stoichiometric amount of potassium nitrate. In the hexagonal form the cerium atoms are arranged along a threefold axis. In hexagonal form the potassium ions are surrounded by nine oxygen atoms. These crystals are orange hexagonal shaped plates. Crystal cells contain three molecules, with a volume of 1063.1Å3 and dimensions of a=13.5737Å c=6.6624Å with a density of 2.767 g/cm3.[32]
In the monoclinic form of K2[Ce(NO3)6], the cerium atoms are in a body centred arrangement, with potassium surrounded by ten oxygen atoms. The density is 2.798 g/cm3 with a cell that contains two molecules with volume 700.9Å3 and dimensions a = 12.707Å b = 6.6858Å c = 8.253Å and β = 91.55°.[22]
Ceric potassium nitrate also has a hydrate with 1.5 mols of water.[10]
- Ceric rubidium nitrate Rb2[Ce(NO3)6] is reddish yellow.[10]
- Ceric caesium nitrate Cs2[Ce(NO3)6] is very insoluble in nitric acid and is bright yellow.[10]
- The thallium double salt cannot be produced because the ceric ion oxidizes thallium(I) to thallium(III).[10]
Divalent metals
- Ceric magnesium nitrate Mg[Ce(NO3)6.8H2O][10]
- Ceric zinc nitrate Zn[Ce(NO3)6.8H2O][10]
- Ceric nickel nitrate Ni[Ce(NO3)6.8H2O][10]
- Ceric cobalt nitrate Co[Ce(NO3)6.8H2O][10]
- Ceric manganese nitrate Mn[Ce(NO3)6.8H2O][10]
Other compounds
- [Ce6O(OH)8(NO3)6(H2O)16]·(NO3)2·2H2O is a hexanuclear cerium oxido and hydroxido complex. It can be dehydrated to form [Ce6O(OH)8(NO3)8].[33]
Proposed application
Cerium magnesium nitrate (also known as cerous magnesium nitrate), is a highly paramagnetic salt, and is a possible refrigerant for use in magnetic refrigeration.[34]
References
- ↑ Garner, J.P.; P.S.J. Heppell (2005). "Cerium nitrate in the management of burns". Burns 31 (5): 539–547. doi:10.1016/j.burns.2005.01.014. ISSN 0305-4179. PMID 15955636.
- ↑ Wassermann, D.; M. Schlotterer, F. Lebreton, J. Levy, M.C. Guelfi (1989). "Use of topically applied silver sulphadiazine plus cerium nitrate in major burns". Burns 15 (4): 257–260. doi:10.1016/0305-4179(89)90045-4. ISSN 0305-4179. PMID 2765148.
- ↑ Latimer, Wendell M.; Hildebrand, Joel H. (1951). Reference Book of Inorganic Chemistry (3 ed.). New York: Macmillan. p. 581.
- ↑ Groth, Paul (1908). Chemische Krystallographie. 2. Leipzig W. Engelmann. p. 131. https://archive.org/details/chemischekrystal02grotuoft.
- ↑ 5.0 5.1 Guillou, N.; Auffredic, J.P.; Louër, M.; Louër, D. (1993). "The Crystal Structure of Hydronium Cerium (III) Nitrate Hydrate, Ce(NO3)5(H3O)2 · H2O". Journal of Solid State Chemistry 106 (2): 295–300. doi:10.1006/jssc.1993.1289. ISSN 0022-4596. Bibcode: 1993JSSCh.106..295G.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 Held, P.; H. Hellwig, S. RuÈhle and L. Bohaty; Rühle, S.; Bohatý, L. (24 Jan 2000). "Polar potassium rare earth nitrates K2[RE(NO3)5(H2O)2] (RE = La, Ce, Pr and Nd). I. Crystal growth and crystal structures". Journal of Applied Crystallography 33 (2): 372–379. doi:10.1107/s0021889800000868. ISSN 0021-8898.
- ↑ Staritscky, Eugene; Donald I Wlaker (29 August 1952). "Optical Properties of some Compounds of Uranium, Plutonium and Related Elements". pp. 20–21. https://www.fas.org/sgp/othergov/doe/lanl/lib-www/la-pubs/00356861.pdf.
- ↑ 8.0 8.1 Wylie, A. W. (1951). "338. Extraction of ceric nitrate by solvents". Journal of the Chemical Society (Resumed): 1474–1480. doi:10.1039/JR9510001474. ISSN 0368-1769.
- ↑ 9.0 9.1 Smith, G. Frederick; C. A. Getz (1940). "Cerate Oxidimetry". Industrial & Engineering Chemistry Analytical Edition 12 (6): 339–340. doi:10.1021/ac50146a012. ISSN 0096-4484.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 Meyer, Richard Jos.; Richard Jacoby (1901). "Die Doppelnitrate des vierwertigen Ceriums und des Thoriums". Zeitschrift für Anorganische Chemie 27 (1): 359–389. doi:10.1002/zaac.19010270131. ISSN 0863-1778. https://zenodo.org/record/1428092.
- ↑ Guillou, N.; Auffrédic, J.P.; Louër, D. (1994). "Synthesis, Crystal Structure, and Thermal Behavior of Cerium(IV) Oxide Nitrate Ce2O(NO3)6(H2O)6 · 2H2O". Journal of Solid State Chemistry 112 (1): 45–52. doi:10.1006/jssc.1994.1262. ISSN 0022-4596. Bibcode: 1994JSSCh.112...45G.
- ↑ Xue, Dongfeng; Siyuan Zhang (1998). "Calculation of nonlinearities of K2Ce(NO3)5• 2H2O and K2La(NO3)5• 2H2O". Molecular Physics 93 (3): 411–415. doi:10.1080/002689798169096. ISSN 0026-8976. Bibcode: 1998MolPh..93..411X.
- ↑ Th. Lange, L. (1861). "Ueber einige neue Cerverbindungen". Journal für Praktische Chemie 82 (1): 129–147. doi:10.1002/prac.18610820119. ISSN 0021-8383. https://zenodo.org/record/1427824.
- ↑ Fock, A. (1894). "Krystallographisch - chemische Untersuchungen". Zeitschrift für Kristallographie 22 (1–6): 29–42. doi:10.1524/zkri.1894.22.1.29. https://zenodo.org/record/1448948.
- ↑ Groth, Paul (1908). Chemische Krystallographie. 2. Leipzig W. Engelmann. https://archive.org/details/chemischekrystal02grotuoft/page/n3.
- ↑ 16.0 16.1 16.2 16.3 Jantsch, G.; S. Wigdorow (1910). "Zur Kenntnis der Doppelnitrate der seltenen Erden. 1. Mitteilung. Über die Doppelnitrate der seltenen Erden mit den Alkalielementen". Zeitschrift für Anorganische Chemie 69 (1): 221–231. doi:10.1002/zaac.19110690117. ISSN 0863-1778. https://zenodo.org/record/1428120.
- ↑ Ebbers, C.A.; L.D. DeLoach, M. Webb, D. Eimerl, S.P. Velsko, D.A. Keszler; Webb, Mark; Eimerl, David; Velsko, Stephan P.; Keszler, Douglas A. (1993). "Nonlinear optical properties of K 2La(NO 3)5.2H2O and K2Ce(NO3) 5.2H2O". IEEE Journal of Quantum Electronics 29 (2): 497–507. doi:10.1109/3.199304. ISSN 0018-9197. Bibcode: 1993IJQE...29..497E.
- ↑ 18.0 18.1 Najafpour, Mahdi; Przemysław Starynowicz (2006). "Diammonium diaquapentanitratocerate(III) dihydrate". Acta Crystallographica Section E 62 (7): i145–i146. doi:10.1107/S1600536806006593. ISSN 1600-5368.
- ↑ Nyquist, Richard A.; Kagel, Ronald O. (1972-03-30). Handbook of Infrared and Raman Spectra of Inorganic Compounds and Organic Salts: Infrared Spectra of Inorganic Compounds. Academic Press. pp. 146–147. ISBN 9780080878522. https://books.google.com/books?id=RhJg4WCbm0gC&pg=PA146. Retrieved 8 February 2014.
- ↑ 20.0 20.1 20.2 "Cerous nitrate, Ce(NO3)3". 2012. http://cerium.atomistry.com/cerous_nitrate.html.
- ↑ Sun, Qiaozhen; Feng Zheng; Xiaodan Sun; Wei Wang (2008). "Bis{4-[(4H-1,2,4-triazol-4-yl)iminomethylpyridinium} diaquapentanitratocerate(III)"]. Acta Crystallographica Section E 65 (1): m124. doi:10.1107/S1600536808043109. ISSN 1600-5368. PMID 21581488.
- ↑ 22.0 22.1 22.2 22.3 Guillou, N.; Auffrédic, J.P.; Louër, D. (1995). "Cerous Potassium Nitrate, K3Ce2(NO3)9". Acta Crystallographica Section C 51 (6): 1032–1034. doi:10.1107/S0108270194014939. ISSN 0108-2701.
- ↑ "Crystal Structure of Diammonium diaquapentanitratocerate(III) dihydrate" (in en). http://crystallography-online.com/structure/2209400.
- ↑ Audebrand, N (1996). "Temperature-dependent X-ray diffraction and crystal structure of CeRb2(NO3)5 · 4H2O". Solid State Ionics 84 (3–4): 323–333. doi:10.1016/0167-2738(96)00082-3. ISSN 0167-2738.
- ↑ 陈达贵; 程文旦; 张浩; 张永春 (16 September 2004). "Synthesis,Crystal Structure and Spectral Properties of [Ce(NO3)5(H2O)2]·2(Hphen)·(H2O)". Chinese Journal of Structural Chemistry 结构化学 23 (8). doi:10.3969/j.issn.0254-5861.2004.08.006.
- ↑ Bertinat, M. P.; D. F. Brewer; J. P. Harrison (1970). "Surface area of powdered cerous magnesium nitrate". Journal of Low Temperature Physics 2 (1): 157–160. doi:10.1007/BF00628109. ISSN 0022-2291. Bibcode: 1970JLTP....2..157B.
- ↑ Abel, W.; A. Anderson; W. Black; J. Wheatley (1966). "Thermal Equilibrium Between Liquid He^{3} and Powdered Cerium Magnesium Nitrate at Very Low Temperatures". Physical Review Letters 16 (7): 273–275. doi:10.1103/PhysRevLett.16.273. ISSN 0031-9007. Bibcode: 1966PhRvL..16..273A.
- ↑ Audebrand, Nathalie; Louër, Daniel (2000). "Cerous sodium nitrate monohydrate, Na2Ce(NO3)5·H2O". Acta Crystallographica Section C 56 (8): 913–915. doi:10.1107/S0108270100006211. ISSN 0108-2701. PMID 10944270.
- ↑ Guillou, N.; Auffrédic, J.P.; Louër, D. (1996). "Thermal Behavior and Crystal Structure of Ceric and Cerous Rubidium Nitrates". Journal of Solid State Chemistry 122 (1): 59–67. doi:10.1006/jssc.1996.0082. ISSN 0022-4596. Bibcode: 1996JSSCh.122...59G.
- ↑ Roser, M.; L. Corruccini (1990). "Magnetic susceptibilities of rare-earth ions in an unusual tetrahedral site". Physical Review B 41 (4): 2359–2368. doi:10.1103/PhysRevB.41.2359. ISSN 0163-1829. PMID 9993972. Bibcode: 1990PhRvB..41.2359R.
- ↑ Thomas A. Beineke; J. Delgaudio (1968). "Crystal structure of ceric ammonium nitrate". Inorg. Chem. 7 (4): 715–721. doi:10.1021/ic50062a020.
- ↑ Guillou, N.; Louër, M.; Auffrédic, J.P.; Louër, D. (1995). "Two Polymorphic Forms of Ceric Potassium Nitrate, K2Ce(NO3)6". Acta Crystallographica Section C 51 (6): 1029–1032. doi:10.1107/S0108270194014927. ISSN 0108-2701.
- ↑ Calvez, Guillaume; Carole Daiguebonne; Olivier Guillou; Florence Le Dret (2009). "A New Series of Anhydrous Lanthanide-Based Octahedral Hexanuclear Complexes". European Journal of Inorganic Chemistry 2009 (21): 3172–3178. doi:10.1002/ejic.200900283. ISSN 1434-1948.
- ↑ Vlasov, A; Guillemette, J; Gervais, G; Szkopek, T (2017). "Magnetic refrigeration with paramagnetic semiconductors at cryogenic temperatures". arXiv:1706.00458 [cond-mat.mes-hall].
For comparison, a commonly used paramagnetic salt for magnetic cryo-refrigeration is cerous magnesium nitrate hydrate ...
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | C | NO−3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||