Chemistry:Corrosion inhibitor
In chemistry, a corrosion inhibitor or anti-corrosive is a chemical compound that, when added to a liquid or gas, decreases the corrosion rate of a material, typically a metal or an alloy, that comes into contact with the fluid.[1] The effectiveness of a corrosion inhibitor depends on fluid composition, quantity of water, and flow regime. Corrosion inhibitors are common in industry, and also found in over-the-counter products, typically in spray form in combination with a lubricant and sometimes a penetrating oil. They may be added to water to prevent leaching of lead or copper from pipes.[2]
A common mechanism for inhibiting corrosion involves formation of a coating, often a passivation layer, which prevents access of the corrosive substance to the metal. Permanent treatments such as chrome plating are not generally considered inhibitors, however: corrosion inhibitors are additives to the fluids that surround the metal or related object.
Types
The nature of the corrosive inhibitor depends on (i) the material being protected, which are most commonly metal objects, and (ii) on the corrosive agent(s) to be neutralized. The corrosive agents are generally oxygen, hydrogen sulfide, and carbon dioxide. Oxygen is generally removed by reductive inhibitors such as amines and hydrazines:
- O
2 + N
2H
4 → 2 H
2O + N
2
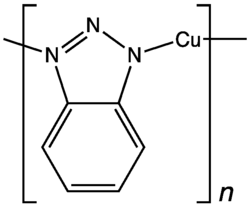
In this example, hydrazine converts oxygen, a common corrosive agent, to water, which is generally benign. Related inhibitors of oxygen corrosion are hexamine, phenylenediamine, and dimethylethanolamine, and their derivatives. Antioxidants such as sulfite and ascorbic acid are sometimes used. Some corrosion inhibitors form a passivating coating on the surface by chemisorption. Benzotriazole is one such species used to protect copper. For lubrication, zinc dithiophosphates are common - they deposit sulfide on surfaces.
The suitability of any given chemical for a task in hand depends on many factors, including their operating temperature.[3]
Illustrative applications
- Volatile amines are used in boilers to minimize the effects of acid. In some cases, the amines form a protective film on the steel surface and, at the same time, act as an anodic inhibitor. An inhibitor that acts both in a cathodic and anodic manner is termed a mixed inhibitor.
- Benzotriazole inhibits the corrosion and staining of copper surfaces.[4]
- Corrosion inhibitors are often added to paints. A pigment with anticorrosive properties is zinc phosphate. Compounds derived from tannic acid or zinc salts of organonitrogens (e.g. Alcophor 827) can be used together with anticorrosive pigments. Other corrosion inhibitors are Anticor 70, Albaex, Ferrophos, and Molywhite MZAP.
- Antiseptics are used to counter microbial corrosion. Benzalkonium chloride is commonly used in oil field industry.
- In oil refineries, hydrogen sulfide can corrode steels so it is removed often using air and amines by conversion to polysulfides.
Tap water
Orthophosphates may be added in tap water treatment systems to prevent leaching of lead and copper from water pipes and reduce the ion content in tap water to safer, legal levels.[2] Polyphosphates can be used to control iron and manganese, which cause discoloration, but do not control lead and copper. The water industry commonly uses a blended-phosphates formulation to deal with both issues.[5] Phosphates convert any leached ions into a layer of scale that acts to separate the metal piping from the water.[6]
Corrosion of drinking water pipes can be influenced by a number of factors such as the pH, buffering capacity, and hardness.[7] Accordingly, other methods control include directly adjusting the pH, adding silicates as an alternative corrosion inhibitor, or adding bicarbonates for buffer.[2]
Phosphate-type inhibitors may cause eutrophication issues downstream or directly encourage algal growth in uncovered, treated water reservoirs. As a result, local water systems may elect to use alternative methods.[8]
In areas with widespread lead and copper piping systems, corrosion control using inhibitors and monitoring techniques is central to water safety. Figures such as the chloride-to-sulfate mass ratio (CSMR) can be used to estimate the risk of corrosion at galvanic connections (i.e. desimilar pipe/solder connections, such as a lead-to-iron transition). The 2014 Flint water crisis was caused by a combination of source water change and a lack of corrosion control. The new, higher-CSMR water not only dissolved lead and iron from the pipes themselves, but also broke up previous layers of lead-containing rusty scale in pipes, allowing them to enter the water supply.[6]
Fuels industry
Corrosion inhibitors are commonly added to coolants, fuels, hydraulic fluids, boiler water, engine oil, and many other fluids used in industry. For fuels, various corrosion inhibitors can be used. Some components include zinc dithiophosphates.[9]
- DCI-4A, widely used in commercial and military jet fuels, acts also as a lubricity additive. Can be also used for gasolines and other distillate fuels.
- DCI-6A, for motor gasoline and distillate fuels, and for U.S. military fuels (JP-4, JP-5, JP-8)
- DCI-11, for alcohols and gasolines containing oxygenates
- DCI-28, for very low-pH alcohols and gasolines containing oxygenates
- DCI-30, for gasoline and distillate fuels, excellent for pipeline transfers and storage, caustic-resistant
- DMA-4 (solution of alkylaminophosphate in kerosene), for petroleum distillates
See also
Useful websites
- "What is corrosion resistance?". http://www.corrosionist.com/what_is_corrosion_resistance.htm.
- "Engineering ToolBox". http://www.engineeringtoolbox.com.
- "Protective Coatings and Inorganic Anti Corrosion Pigments". http://www.astm.org/DIGITAL_LIBRARY/MNL/PAGES/MNL12208M.htm.
- "Electro Galvanized Steel | Rolled Steel Products « Rolled Steel Products". http://www.rolledsteel.com/electro-galvanized-steel/.
- "Anti corrosion coatings". https://www.performancecoatings.org/solutions/anti-corrosion-coatings/.
- "Corrosion Resistance - DECC Company". http://www.decc.com/corrosion_protection.php.*"Nitriding for Corrosion and Wear Fatigue Resistance" (in en). Corrosionpedia. https://www.corrosionpedia.com/2/1513/prevention/surface-modification/nitriding-for-corrosion-and-wear-fatigue-resistance.
References
- ↑ Hubert Gräfen, Elmar-Manfred Horn, Hartmut Schlecker, Helmut Schindler "Corrosion" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH: Weinheim, 2002. doi:10.1002/14356007.b01_08
- ↑ 2.0 2.1 2.2 "Optimal Corrosion Control Treatment Evaluation Technical Recommendations for Primacy Agencies and Public Water Systems". 2019. https://www.epa.gov/sites/production/files/2019-07/documents/occtmarch2016updated.pdf.
- ↑ Ma, I. A. Wonnie; Ammar, Sh.; Kumar, Sachin S. A.; Ramesh, K.; Ramesh, S. (2022-01-01). "A concise review on corrosion inhibitors: types, mechanisms and electrochemical evaluation studies" (in en). Journal of Coatings Technology and Research 19 (1): 241–268. doi:10.1007/s11998-021-00547-0. ISSN 1935-3804. https://doi.org/10.1007/s11998-021-00547-0.
- ↑ M. Finšgarand and I. Milošev "Inhibition of copper corrosion by 1,2,3-benzotriazole: A review" Corrosion Science 2010, Volume 52, Pages 2737-2749 doi:10.1016/j.corsci.2010.05.002
- ↑ "Why do water systems add phosphate to drinking water? What are the health effects of drinking water containing phosphates?". https://www.epa.gov/lead/why-do-water-systems-add-phosphate-drinking-water-what-are-health-effects-drinking-water.
- ↑ 6.0 6.1 Pieper, Kelsey J.; Tang, Min; Edwards, Marc A. (21 February 2017). "Flint Water Crisis Caused By Interrupted Corrosion Control: Investigating "Ground Zero" Home". Environmental Science & Technology 51 (4): 2007–2014. doi:10.1021/acs.est.6b04034. PMID 28145123. Bibcode: 2017EnST...51.2007P.
- ↑ "Drinking Water Pipe Systems | Engineering | Community Water Fluoridation | Division of Oral Health | CDC" (in en-us). 5 November 2018. https://www.cdc.gov/fluoridation/engineering/corrosion.htm.
- ↑ "Investigation of Potential Environmental Impacts due to the use of Phosphate-based Corrosion Inhibitors in the District of Columbia". July 22, 2004. https://archive.epa.gov/region03/dclead/web/pdf/impacts_report_final.pdf.
- ↑ Octel-Starreon Refinery Fuel Additives Corrosion Inhibitors for hydrocarbon fuels - corrosion inhibitor and corrosion protection to fuel distribution system
External links
- Corrosion control informations and documents
- Developing corrosion inhibitor models – A paper on corrosion inhibition modeling
- Corrosion inhibition, Norman Hackerman,(August, 2006), Electrochemistry Encyclopedia[|permanent dead link|dead link}}]
 |