Chemistry:Cinchophen
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
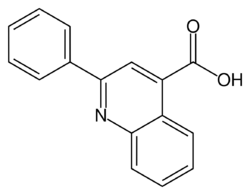
| Formula | C16H11NO2 |
| Molar mass | 249.269 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cinchophen (trade names Atophan, Quinophan, and Phenaquin) is an analgesic drug that was first produced by Doebner & Gieskel in 1887, it was commercially introduced in 1908 as a treatment for gout. This drug is still used, in combination with Prednisolone, by veterinarians to treat arthritis in animals. It can be prepared starting from anilin, benzaldehyde and pyruvic acid in absolute ethanol.[1] Use of this drug in humans ceased in the 1930s due to the discovery that cinchophen can cause serious liver damage.[2] There is some evidence that it stimulates C-Fos.[3]
History
Cinchophen was first introduced in 1908 as 'Atophan' and used for the treatment of gout and arthritis.[4] It was efficacious at reducing the buildup of uric acid and became a popular medicine. Further studies in 1911 and 1912 were successful in isolating metabolites of cinchophen from human urine. During the 1920s, cases of severe adverse effects such as liver damage and cirrhosis had been clinically observed; the first case of jaundice was recorded in 1923 and the first fatality occurred in 1925. Approximately half of all patients experienced toxic effects and by 1932 there were 32 drugs available that contained cinchophen or its derivatives as their active ingredients,[5] experiments were conducted to determine the drugs pharmacology [6] but the mechanism of drug induced liver injury remained unclear. Cinchophen was considered to be too dangerous for human use in most countries but has applications in veterinary medicine as a treatment for osteoarthritis in dogs.
Available forms
Cinchophen can be administered orally in tablet form or intravenously in liquid form.[7]
Toxicity and side-effects
Cinchophen toxicity may present 6 to 12 hours after administration. Symptoms can include hyperventilation, hyperthermia (fever), gastrointestinal discomfort, diarrhoea, hives, vomiting, delirium, hepatitis,[8] jaundice, anorexia, convulsions, coma and death.[7] Fatty degeneration of the heart and kidneys, necrosis of hepatic cells [9] in addition to yellow atrophy of the liver have been recorded in autopsy findings.
Treatment
Cinchophen administration should be immediately ceased if symptoms occur. There is no cure for acute cinchophen poisoning but early diagnosis and symptomatic treatments may be effective. Calcium lactate may be used to treat hives and oral or intravenous glucose can relieve gastrointestinal symptoms. If glucose is given, insulin may be needed to protect the liver from further damage. Oral alkali treatments can combat acidosis and anti-emetics may be required to manage nausea and vomiting.[8]
Veterinary medicine
Cinchophen is the main active ingredient in prednoleucotropin (PLT), a medication used in the treatment of osteoarthritis in dogs.[10] PLT may produce analgesic and anti-pyretic effects at higher doses. Osteoarthritic dogs treated with PLT have shown significant improvement of joint mobility, stiffness, lameness and weight bearing capacity.[11] When administered correctly, PLT has shown similar clinical efficacy to phenylbutazone. The effects of PLT toxicity in dogs are similar to cinchophen toxicity in humans and include hepatotoxicity and gastric ulceration.
References
- ↑ Intermediates for Organic Synthesis. I. K. International. 2005. pp. 262. ISBN 978-81-88-237-33-3.
- ↑ "[Toxic hepatitis from cinchophen: report of 3 cases]" (in es). Medicina Clinica 97 (3): 104–106. June 1991. PMID 1679861.
- ↑ "Neuronal expression of Fos protein in the paraventricular nucleus of the hypothalamus after i.p. injection of ulcergenic cinchophen". Neuroscience Letters 172 (1–2): 55–58. May 1994. doi:10.1016/0304-3940(94)90661-0. PMID 7916144.
- ↑ "The effect of salicylate and cinchophen on enzymes and metabolic processes". The Biochemical Journal 36 (10–12): 706–728. December 1942. doi:10.1042/bj0360706. PMID 16747500.
- ↑ "Cinchophen—Is There a Safe Method of Administration?" (in en). Journal of the American Medical Association 107 (10): 760–764. 1936-09-05. doi:10.1001/jama.1936.02770360006003. ISSN 0002-9955.
- ↑ "Cinchophen Poisoning: An Attempt to Produce Toxic Cirrhosis of the Liver in Rats" (in en). Archives of Internal Medicine 49 (2): 215–220. 1932-02-01. doi:10.1001/archinte.1932.00150090045005. ISSN 0730-188X.
- ↑ 7.0 7.1 Book of poisons : a guide for writers. Cincinnati, Ohio: Writer's Digest Books.. 2007. ISBN 9781599634401. OCLC 756774729.
- ↑ 8.0 8.1 "Toxic Hepatitis Due to Cinchophen: A Report of Three Cases". Canadian Medical Association Journal 26 (2): 170–174. February 1932. PMID 20318604.
- ↑ "Cinchophen Administration - Jaundice as an Untoward Effect: Report of Cases". California and Western Medicine 37 (1): 30–33. July 1932. PMID 18742180.
- ↑ "Chronic pain: what are the options when NSAID treatment is inadequate?" (in en). Companion Animal 19 (4): 212–217. 2014. doi:10.12968/coan.2014.19.4.212. ISSN 2053-0889.
- ↑ "Pharmacokinetics and clinical efficacy of a cinchophen and prednisolone combination in the dog" (in en). Journal of Small Animal Practice 32 (2): 53–58. 1991. doi:10.1111/j.1748-5827.1991.tb00913.x. ISSN 0022-4510.
 |
