Chemistry:Guaifenesin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɡwaɪˈfɛnɪsɪn/[1] |
| Trade names | Mucinex, others |
| Other names | Glyceryl guaiacolate |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682494 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Kidney |
| Elimination half-life | 1–5 hours[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
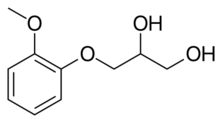
| Formula | C10H14O4 |
| Molar mass | 198.218 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Guaifenesin, also known as glyceryl guaiacolate, is an expectorant medication taken by mouth and marketed as an aid to eliminate sputum from the respiratory tract. Chemically, it is an ether of guaiacol and glycerine. It may be used in combination with other medications.[3] A 2014 study found that guaifenesin has no effect on sputum production or clearance in upper respiratory infections.[4][5]
Side effects may include dizziness, sleepiness, skin rash, and nausea.[3] While it has not been properly studied in pregnancy, it appears to be safe.[6] It is believed to work by making airway secretions more liquid.[3]
Guaifenesin has been used medically since at least 1933.[7] It is available as a generic medication and over the counter.[3][6] In 2021, it was the 288th most commonly prescribed medication in the United States, with more than 600,000 prescriptions.[8][9]
Medical use
Guaifenesin is used to try to help with coughing up thick mucus, and is sometimes combined with the antitussive (cough suppressant) dextromethorphan, such as in Mucinex DM or Robitussin DM.[10] It is also combined with ephedrine in Primatene and Bronkaid tablets for symptomatic relief of asthma.
A Cochrane review identified only three clinical trials assessing guaifenesin for the treatment of acute cough, with one finding significant benefit and the other two trials finding that it was not effective.[11]
Side effects
Although generally well-tolerated, side-effects of guaifenesin may include an allergic reaction (rare), nausea, vomiting, dizziness or headache.[12][13]
Pharmacology
Mechanism of action
Guaifenesin is thought to act as an expectorant by increasing the volume and reducing the viscosity of secretions in the trachea and bronchi. It may aid in the flow of respiratory tract secretions, allowing ciliary movement to carry the loosened secretions upward toward the pharynx.[14] Thus, it may increase the efficiency of the cough reflex and facilitate removal of the secretions.
History
Similar medicines derived from the guaiac tree were in use as a generic remedy by American indigenous peoples when explorers reached North America in the 16th century. The Spanish encountered guaiacum wood "when they conquered Santo Domingo; it was soon brought back to Europe, where it acquired an immense reputation in the sixteenth century as a cure for syphilis and certain other diseases..."[15]
The 1955 edition of the Textbook of Pharmacognosy states: "Guaiacum has a local stimulant action which is sometimes useful in sore throat. The resin is used in chronic gout and rheumatism, whilst the wood is an ingredient in the compound concentrated solution of sarsaparilla, which was formerly much used as an alternative in syphilis."[15]
In the US, guaifenesin was first approved by the Food and Drug Administration (FDA) in 1952. Although previously deemed "Generally Regarded as Safe" in its original approval, the drug received a New Drug Application for the extended-release version, which received approval on 12 July 2002.[16] Because of this, the FDA then issued letters to other manufacturers of timed-release guaifenesin to stop marketing their unapproved versions, leaving Adams Respiratory Therapeutics in control of the market. In 2007, Adams was acquired by Reckitt Benckiser.[17][18] The drug is now sold over-the-counter by many companies, alone and in combination.[19]
Society and culture
Brand names
Guaifenesin is taken by mouth,[3] and is supplied as a tablet, a capsule, an extended-release (long-acting) tablet, dissolving granules, and a syrup.[13] It is available under many brand names, as either the sole active ingredient or part of a combination drug.[13] Drugs combined with guaifenesin in over-the-counter preparations include the cough-suppressant dextromethorphan, analgesics such as paracetamol/acetaminophen, and decongestants such as ephedrine, pseudoephedrine, or phenylephrine.[13]
Economics
In 2014, sales of guaifenesin were estimated to be approximately $135 million per year.[5]
Veterinary use
Guaifenesin's neurological properties first became known in the late 1940s. Guaifenesin is a centrally acting muscle relaxant used routinely in large-animal veterinary surgery. Guaifenesin is used in combination with, for example, ketamine, since guaifenesin does not provide analgesia or produce unconsciousness.[20][21]
References
- ↑ "Guaifenesin Definition & Meaning". Merriam-Webster. https://www.merriam-webster.com/medical/guaifenesin.
- ↑ "Determination of guaifenesin in human plasma by liquid chromatography in the presence of pseudoephedrine". J Pharm Biomed Anal 11 (9): 803–808. 1993. doi:10.1016/0731-7085(93)80072-9. PMID 8218524.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Guaifenesin: Monograph for Professionals". Drugs.com, American Society of Health-System Pharmacists. 23 January 2023. https://www.drugs.com/monograph/guaifenesin.html.
- ↑ "Guaifenesin has no effect on sputum volume or sputum properties in adolescents and adults with acute respiratory tract infections". Respiratory Care 59 (5): 631–636. May 2014. doi:10.4187/respcare.02640. PMID 24003241.
- ↑ 5.0 5.1 "Is extended-release guaifenesin no better than a placebo?". Respir Care 59 (5): 788–9. May 2014. doi:10.4187/respcare.03319. PMID 24789023.
- ↑ 6.0 6.1 (in en) The Complete Guide to Medications During Pregnancy and Breastfeeding: Everything You Need to Know to Make the Best Choices for You and Your Baby. St. Martin's Press. 2013. p. PT282. ISBN 9781250037206. https://archive.org/details/completeguidetom0000wein_t9k5.
- ↑ Veterinary Pharmacology and Therapeutics. John Wiley & Sons. 2013. p. 287. ISBN 9781118685907. https://books.google.com/books?id=xAPa4WDzAnQC&pg=PA287.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Guaifenesin – Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Guaifenesin.
- ↑ "Guaifenesin DM". WebMD.com. http://www.webmd.com/drugs/mono-704-GUAIFENESIN%2fDEXTROMETHORPHAN+-+ORAL.aspx?drugid=5508&drugname=Guaifenesin+DM+Oral&source=0.
- ↑ "Over-the-counter (OTC) medications for acute cough in children and adults in community settings". Cochrane Database Syst Rev 2014 (11): CD001831. November 2014. doi:10.1002/14651858.CD001831.pub5. PMID 25420096.
- ↑ "Guaifenesin Side Effects". Drugs.com. 3 July 2023. https://www.drugs.com/sfx/guaifenesin-side-effects.html.
- ↑ 13.0 13.1 13.2 13.3 "Guaifenesin". MedlinePlus, United States National Library of Medicine. 15 January 2022. https://www.nlm.nih.gov/medlineplus/druginfo/meds/a682494.html. Retrieved 30 July 2023.
- ↑ Gutierrez, K. (2007). Pharmacotherapeutics: Clinical Reasoning in Primary Care. W.B. Saunders Co.
- ↑ 15.0 15.1 Textbook of Pharmacognosy. 1955. https://archive.org/details/textbookofpharma00wall.
- ↑ "Drug Approval Package: Mucinex (Guaifenesin) NDA #21-282". 25 November 2002. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2002/21-282_Mucinex.cfm.
- ↑ "Announcements RB Press release - 10/12/2007". http://www.rb.com/site/RKBR/Templates/MediaInvestorsGeneral2.aspx?pageid=262&cc=GB.
- ↑ "FDA Bumps Phlegm-Fighters From Market". The Wall Street Journal. 25 May 2007. https://blogs.wsj.com/health/2007/05/25/fda-bumps-phlegm-fighters-from-market/.
- ↑ "Guaifenesin (Oral Route) Description and Brand Names". https://www.mayoclinic.org/drugs-supplements/guaifenesin-oral-route/description/drg-20068720.
- ↑ "Centrally Acting Muscle Relaxants". Lumb and Jones' Veterinary Anesthesia and Analgesia (2nd ed.). Blackwell Publishing. 2007.
- ↑ "Balanced anesthesia and constant-rate infusions in horses". Vet Clin North Am Equine Pract 29 (1): 89–122. Apr 2013. doi:10.1016/j.cveq.2012.11.004. PMID 23498047.
External links
- "F.D.A. Study Worries Makers of Drugs". 20 October 1981. https://www.nytimes.com/1981/10/20/business/fda-study-worries-makers-of-drugs.html.
 |

