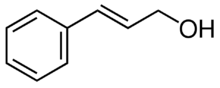
Chemistry:Cinnamyl alcohol
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-3-Phenylprop-2-en-1-ol | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10O | |
| Molar mass | 134.17 g/mol |
| Density | 1.0397 g/cm3 at 35 °C |
| Melting point | 33 °C |
| Boiling point | 250 °C |
| Slightly | |
| Solubility | soluble in ethanol, acetone, dichloromethane |
| -87.2·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H317 | |
| P261, P272, P280, P302+352, P321, P333+313, P363, P501 | |
| Flash point | 126°C |
| Related compounds | |
Related compounds
|
Cinnamic acid; Cinnamaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cinnamyl alcohol or styron[1] is an organic compound that is found in esterified form in storax, Balsam of Peru, and cinnamon leaves. It forms a white crystalline solid when pure, or a yellow oil when even slightly impure. It can be produced by the hydrolysis of storax.
Cinnamyl alcohol has a distinctive odour described as "sweet, balsam, hyacinth, spicy, green, powdery, cinnamic" and is used in perfumery[2] and as a deodorant.
Cinnamyl alcohol is naturally occurrent only in small amount, so its industrial demand is usually fulfilled by chemical synthesis starting from cinnamaldehyde.[3]
Properties
The compound is a solid at room temperature, forming colourless crystals that melt upon gentle heating. As is typical of most higher-molecular weight alcohols, it is sparingly soluble in water at room temperature, but highly soluble in most common organic solvents.
Safety
Cinnamyl alcohol has been found to have a sensitising effect on some people[4][5] and as a result is the subject of a Restricted Standard issued by IFRA (International Fragrance Association).
Glycosides
Rosarin and rosavin are cinnamyl alcohol glycosides isolated from Rhodiola rosea.
References
- ↑ 1.0 1.1 Chemical News and Journal of Industrial Science, Volumes 27-28, Sir William Crookes, page 126
- ↑ "cinnamyl alcohol 104-54-1". thegoodscentscompany.com. http://www.thegoodscentscompany.com/data/rw1003291.html. Retrieved 26 July 2015.
- ↑ Zucca, P; Littarru, M; Rescigno, A; Sanjust, E (May 2009). "Cofactor recycling for selective enzymatic biotransformation of cinnamaldehyde to cinnamyl alcohol.". Bioscience, Biotechnology, and Biochemistry 73 (5): 1224–6. doi:10.1271/bbb.90025. PMID 19420690.
- ↑ "Food and Chemical Toxicology". RIFM. 2007. http://www.rifm.org/doc/Food%20%26%20Chem%20Tox%20RIFM%20Spec%20Suppl%20122007.pdf.
- ↑ Survey and health assessment of chemical substances in massage oils
- Merck Index, 11th Edition, 2305.
 |

