Chemistry:Citral
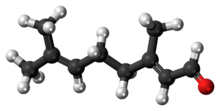
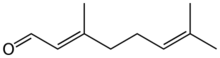 Geranial
| |
| |
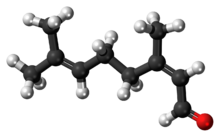
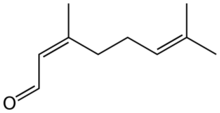 Neral
| |
| |
| Names | |
|---|---|
| IUPAC name
3,7-dimethylocta-2,6-dienal
| |
| Other names
citral
geranialdehyde | |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2810 |
| |
| |
| Properties | |
| C10H16O | |
| Molar mass | 152.24 g/mol |
| Appearance | Pale yellow liquid |
| Odor | Lemon like |
| Density | 0.893 g/cm3 |
| Boiling point | 229 °C (444 °F; 502 K) |
| Vapor pressure | 0.22 mmHg (20 °C) |
| −98.9×10−6 cm3/mol | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H317 | |
| P261, P264, P272, P280, P302+352, P321, P332+313, P333+313, P362, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 91 °C (196 °F; 364 K) |
| Related compounds | |
Related alkenals
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Citral is an acyclic monoterpene aldehyde. Being a monoterpene, it is made of two isoprene units. Citral is a collective term which covers two geometric isomers that have their own separate names; the E-isomer is named geranial (trans-citral; α-citral[2]) or citral A. The Z-isomer is named neral (cis-citral; β-citral[2]) or citral B. These stereoisomers occur as a mixture, not necessarily racemic; e.g. in essential oil of Australian ginger, the neral to geranial ratio is 0.61.[3]
Occurrence
Citral is present in the volatile oils of several plants, including lemon myrtle (90–98%), Litsea citrata (90%), Litsea cubeba (70–85%), lemongrass (65–85%), lemon tea-tree (70–80%), Ocimum gratissimum (66.5%), Lindera citriodora (about 65%), Calypranthes parriculata (about 62%), petitgrain (36%), lemon verbena (30–35%), lemon ironbark (26%), lemon balm (11%), lime (6–9%), lemon (2–5%), and orange.[4][5][6] Further, in the lipid fraction (essential oil) of Australian ginger (51–71%)[3] Of the many sources of citral, the Australian myrtaceous tree, lemon myrtle, Backhousia citriodora F. Muell. (of the family Myrtaceae), is considered superior.[7]
Uses
Citral has a strong lemon (citrus) scent and is used as an aroma compound in perfumery. It is used to fortify lemon oil. (Nerol, another perfumery compound, has a less intense but sweeter lemon note.) The aldehydes citronellal and citral are considered key components responsible for the lemon note with citral preferred.[7]
It also has pheromonal effects in acari and insects.[8][9]
Citral is used in the synthesis of vitamin A, lycopene, ionone and methylionone, and to mask the smell of smoke.
The herb Cymbopogon citratus has shown promising insecticidal and antifungal activity against storage pests.[10]
Food additive
Citral is commonly used as a food additive ingredient.[11]
It has been tested (2016) in vitro against the food-borne pathogen Cronobacter sakazakii.[12]
Medical exploration
In a report (1997), citral is mentioned as cytotoxic to P(388) mouse leukaemia cells.[10] It has strong antimicrobial qualities.[13]
Adverse effects
Two studies showed 1–1.7% of people to be allergic to citral, with allergies frequently reported.[citation needed] Citral on its own is strongly sensitizing to allergies; the International Fragrance Association recommends that citral only be used in association with substances that prevent a sensitizing effect.[citation needed] Citral has been extensively tested, with no known genotoxicity or carcinogenic effect.[14]
See also
- Citronellal
- Geraniol
- Limonene
- Nerol
- Vaporizer
References
- ↑ Citral, The Merck Index, 12th Edition.
- ↑ 2.0 2.1 Waghulde, S.; Parmar, P.; Mule, J.; Pashte, D.; Patil, B.; Modhale, N.; Gorde, N.; Kharche, A. et al. (2020). "Lead Finding from Plant Cymbopogon Citratus with Immunomodulator Potentials through in Silico Methods" (in en). Chemistry Proceedings 3 (1): 77. doi:10.3390/ecsoc-24-08302. ISSN 2673-4583.
- ↑ 3.0 3.1 Zachariah, T. J.; Parthasarathy, V. A.; Chempakam, B. (2008). Chemistry of spices. Wallingford: CABI. p. 76. ISBN 9781845934057. OCLC 1120264204.
- ↑ Fenaroli, G.; Furia, T.E.; Bellanca, N.. Handbook of Flavor Ingredients. ISBN 0-87819-532-7.
- ↑ Lawless, J. (2 November 1995). The Illustrated Encyclopedia of Essential Oils. Element. ISBN 1-85230-661-0.
- ↑ "The Aromatic Plant Project". http://www.aromaticplantproject.com/articles_archive/lemon_citrus_scented_oils.html.
- ↑ 7.0 7.1 Southwell, Ian (9 July 2021). "Backhousia citriodora F. Muell. (Lemon Myrtle), an Unrivalled Source of Citral". Foods 10 (7): 1596. doi:10.3390/foods10071596. PMID 34359465.
- ↑ Kuwahara, Yasumasa; Suzuki, Hiroshi; Matsumoto, Katsuhiko; Wada, Yoshitake (1983). "Pheromone Study on Acarid Mites. XI. Function of Mite Body as Geometrical Isomerization and Reduction of Citral (the Alarm Pheromone)". Applied Entomology and Zoology 18 (1): 30–39. doi:10.1303/aez.18.30.
- ↑ Robacker, D.C.; Hendry, L.B. (1977). "Neral and geranial: components of the sex pheromone of the parasitic wasp, Itoplectis conquisitor". Journal of Chemical Ecology 3 (5): 563–577. doi:10.1007/BF00989077.
- ↑ 10.0 10.1 Dubey, N. K.; Takeya, Koichi; Itokawa, Hideji (1997). "Citral: A cytotoxic principle isolated from the essential oil of Cymbopogon citratus against P388 leukaemia cells". Current Science 73 (1): 22–24.
- ↑ Liao, Pei-Chun; Yang, Tsung-Shi; Chou, Ju-Ching; Chen, Jie; Lee, Shu-Ching; Kuo, Yueh-Hsiung; Ho, Chen-Lung; Chao, Louis Kuo-Ping (1 December 2015). "Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour". Journal of Functional Foods 19: 248–258. doi:10.1016/j.jff.2015.09.034.
- ↑ Shi, Chao; Song, Kaikuo; Zhang, Xiaorong; Sun, Yi; Sui, Yue; Chen, Yifei; Jia, Zhenyu; Sun, Huihui et al. (14 July 2016). "Antimicrobial Activity and Possible Mechanism of Action of Citral against Cronobacter sakazakii". PLOS ONE 11 (7): e0159006. doi:10.1371/journal.pone.0159006. PMID 27415761. Bibcode: 2016PLoSO..1159006S.
- ↑ Onawunmi, Grace O. (September 1989). "Evaluation of the antimicrobial activity of citral". Letters in Applied Microbiology 9 (3): 105–108. doi:10.1111/j.1472-765X.1989.tb00301.x.
- ↑ Andersen, Dorthe N.; Holmberg, Rikke D.; Larsen, Jette R.; Søborg, Inge; Cohr, Karl-Heinz (2006). Survey and health assessment of chemical substances in massage oils. Survey of Chemical Substances in Consumer Products, No. 78. Danish Toxicology Centre. https://www2.mst.dk/udgiv/publications/2006/87-7052-278-2/pdf/87-7052-279-0.pdf.
External links
 |

