Biology:Lemon balm
| Lemon balm | |
|---|---|

| |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Lamiales |
| Family: | Lamiaceae |
| Genus: | Melissa |
| Species: | M. officinalis
|
| Binomial name | |
| Melissa officinalis | |
Lemon balm (Melissa officinalis)[note 1] is a perennial herbaceous plant in the mint family and native to south-central Europe, the Mediterranean Basin, Iran, and Central Asia, but now naturalised elsewhere.
It grows to a maximum height of 1 m (3 ft 3 in). The leaves have a mild lemon scent. During summer, small white flowers full of nectar appear. It is not to be confused with bee balm (genus Monarda), although the white flowers attract bees, hence the genus Melissa (Greek for "honey bee").
The leaves are used as a herb, in teas and also as a flavouring. The plant is used to attract bees for honey production. It is grown as an ornamental plant and for its oil (to use in perfumery). Lemon balm has been cultivated at least since the 16th century.
Description
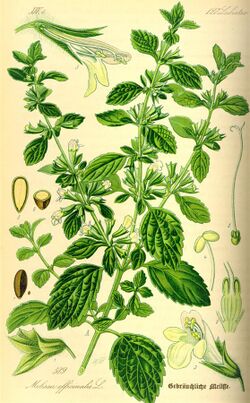
Lemon balm (Melissa officinalis) is a perennial herbaceous plant in the mint family Lamiaceae,[1] and native to south-central Europe, the Mediterranean Basin, Iran, and Central Asia, but now naturalized in the Americas and elsewhere.[5] The second name, officinalis (Latin, 'of the shop'), originates from the use of the herb by apothecaries, who sold herbal remedies directly to their customers.[6]
Lemon balm plants grow bushy and upright to a maximum height of 100 cm (39 in). The heart-shaped leaves are 2–8 centimetres (0.79–3.15 in) long, and have a rough, veined surface. They are soft and hairy with scalloped edges, and have a mild lemon scent. During summer, small white or pale pink flowers appear. The plants live for ten years; the crop plant is replaced after five years to allow the ground to rejuvenate.[7]
Historical uses
The use of lemon balm can be dated to over 2000 years ago through the Ancient Greece and the Romans. It is mentioned by the Greek polymath Theophrastus in his Historia Plantarum, written in c.300 BC,[8] as "bee-leaf" (μελισσόφυλλον).[9] Lemon balm was formally introduced into Europe in the 7th century, from which its use and domestication spread.[8] Its use in the Middle Ages is noted by herbalists, writers, philosophers, and scientists.
Lemon balm was a favourite plant of the Tudors, who scattered the leaves across their floors.[10] It was in the herbal garden of the English botanist John Gerard in the 1590s,[11][page needed] who considered it especially good for feeding and attracting honeybees.[12] Especially cultivated for honey production, according to the authors Janet Dampney and Elizabeth Pomeroy, "bees were thought never to leave a garden in which it was grown".[10] It was introduced to North America by the first colonists from Europe; it was cultivated in the Gardens of Monticello, designed by the American statesman Thomas Jefferson.[13]
The English botanist Nicholas Culpeper considered lemon balm to be ruled by the planet Jupiter in Cancer, and suggested it to be used for "weak stomachs", to cause the heart to become "merry", to help digestion, to open "obstructions of the brain", and to expel "melancholy vapors" from the heart and arteries.[14]
In traditional Austrian medicine, M. officinalis leaves have been prescribed as a herbal tea, or as an external application in the form of an essential oil.[15][page needed]
Current uses
Lemon balm is the main ingredient of carmelite water, which is sold in German pharmacies.[16]
The plant is grown and sold as an ornamental plant, and for attracting bees. The essential oil is used as a perfume ingredient.[17] It is used in toothpaste.[18]
Lemon balm is used as a flavouring[17] in ice cream and herbal teas, often in combination with other herbs such as spearmint. The leaves are not dried when used for tea. It is a common addition to peppermint tea, mostly because of its complementing flavor.[citation needed] Lemon balm is also used with fruit dishes or candies. It can be used in fish dishes and is the main ingredient in lemon balm pesto.[19]: 15–16 Its flavour comes from geraniol (3–40%), neral (3–35%), geranial (4–85%) (both isomers of citral), (E)-caryophyllene (0–14%), and citronellal (1–44%).[20] It is also one of the ingredients in Spreewald gherkins.[21]
Cultivation

Melissa officinalis is native to Europe, central Asia and Iran, but is now naturalized around the world.[3][19] It grows easily from seed, preferring rich, moist soil.[22]
Lemon balm seeds require light and a minimum temperature of 20 °C (68 °F) to germinate. The plant grows in clumps and spreads vegetatively (a new plant can grow from a fragment of the parent plant), as well as by seed. In mild temperate zones, the plant stems die off at the start of the winter, but shoot up again in spring. Lemon balm grows vigorously.[23]
As of 1992[update], Hungary, Egypt, and Italy are the major producing countries of lemon balm.[4] The leaves are harvested by hand in June and August in the northern hemisphere, on a day when the weather is dry, to prevent the crop from turning black if damp.[7]
The cultivars of M. officinalis include:
- M. officinalis 'Citronella'
- M. officinalis 'Lemonella'
- M. officinalis 'Quedlinburger'
- M. officinalis 'Lime'
- M. officinalis 'Mandarina'
- M. officinalis 'Variegata'
- M. officinalis 'Aurea'
- M. officinalis 'Quedlinburger Niederliegende', an improved variety bred for high essential oil content.[citation needed]
Essential oil production
Ireland is a major producer of lemon balm essential oil, which has a pale yellow colour and a lemon scent.[4] The essential oil is commonly co-distilled with lemon oil, citronella oil or other essential oils.[24] Yields are low; 0.014% for fresh leaves and 0.112% for dried leaves.[4]

Chemistry
Lemon balm contains eugenol, tannins, and terpenes.[25]
| Component | minimum % | maximum % |
|---|---|---|
| Methyl Heptenone | 2.2 | 8.6 |
| Citronellal | 1.0 | 8.4 |
| Linalool | 0.5 | 2.7 |
| Neral | 19.6 | 36.1 |
| Geranial | 25.3 | 47.5 |
| Geranyl acetate | 1.2 | 6.2 |
| Carophyllene | 1.9 | 9.7 |
| Carophyllene oxide | 0.5 | 9.0 |
Notes
References
- ↑ 1.0 1.1 1.2 Bahtiyarca Bagdat & Coşge 2006, p. 116.
- ↑ Chisholm 1911, p. 283.
- ↑ 3.0 3.1 "Melissa officinalis". Natural Resources Conservation Service PLANTS Database. USDA. https://plants.usda.gov/core/profile?symbol=MEOF2.
- ↑ 4.0 4.1 4.2 4.3 4.4 Axtell & Fairman 1992, p. 211.
- ↑ "Melissa officinalis L., Sp. Pl.: 592 (1753)". Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/namedetail.do?name_id=124103.
- ↑ Dampney & Pomeroy 1985, p. 11.
- ↑ 7.0 7.1 Axtell & Fairman 1992, p. 212.
- ↑ 8.0 8.1 Kennedy et al. 2002.
- ↑ Theophrastus 1916, p. 464.
- ↑ 10.0 10.1 Dampney & Pomeroy 1985, p. 12.
- ↑ Gerard 1876.
- ↑ Grieve 1971, p. 76.
- ↑ Zirkle 2001, pp. 84–85.
- ↑ Culpepper 1814, pp. 15–16.
- ↑ Vogl et al. 2013.
- ↑ Hiller, Sabine (6 September 2010). "Using lemon balm in the kitchen". The Mayo News. https://www.mayonews.ie/index.php?option=com_content&view=article&id=10742:food-using-lemon-balm-in-the-kitchen&catid=74:tasting&Itemid=100028.
- ↑ 17.0 17.1 "Taxon: Melissa officinalis L.". USDA: U.S. National Plant Germplasm System. https://npgsweb.ars-grin.gov/gringlobal/taxonomydetail.aspx?24036.
- ↑ Dousti 2012, p. 88.
- ↑ 19.0 19.1 Herb Society of America. 2007 Lemon Balm: An Herb Society of America Guide
- ↑ Setzer 2009, p. 1309.
- ↑ "Lemon Balm - Melissa officinalis - Herb Seeds from Victory Seeds®". https://www.victoryseeds.com/melissa_officinalis.html.
- ↑ Dampney & Pomeroy 1985, p. 36.
- ↑ "Herbal Guide to Lemon Balm: Grow, Harvest, and Use a Lemon Balm Plant" (in en-US). 2021-03-24. https://gardentherapy.ca/lemon-balm/.
- ↑ Sarkic, Asja; Stappen, Iris (March 2018). "Essential Oils and Their Single Compounds in Cosmetics—A Critical Review" (in en). Cosmetics 5 (1): 11. doi:10.3390/cosmetics5010011. ISSN 2079-9284.
- ↑ Ehrlich, Steven D. (January 2, 2015). "Lemon balm". University of Maryland Medical Center. http://umm.edu/health/medical/altmed/herb/lemon-balm.
- ↑ Axtell & Fairman 1992, p. 213.
Works cited
- Axtell, B.L.; Fairman, R.M. (1992). "Melissa officinalis". Minor Oil Crops. Rome: Food and Agriculture Organization of the United Nations. ISBN 978-92-5-103128-5. https://archive.org/details/minoroilcrops0000axte.
- Bahtiyarca Bagdat, Reyhan; Coşge, Belgin (January 2006). "The Essential Oil of Lemon Balm (Melissa officinalis L.): its components and using fields". Journal of the Faculty of Agriculture, OMU 21: 116–121. https://www.researchgate.net/publication/228107487. Retrieved 2019-02-09.
- Culpepper, Nicholas (1814). Culpeper's Complete Herbal. London: Richard Evans. OCLC 1029959639. https://archive.org/details/b22011778.
- Dampney, Janet; Pomeroy, Elizabeth (1985). All About Herbs. New York: Exeter Books. ISBN 978-06710-7-536-1. https://archive.org/details/allaboutherbs0000damp_o1j4.
- Dousti, Mashta (2012). "Evidence-based Traditional Persian Medicine". in Rastogi, Sanjeev; Chiappelli, Francesco; Ramchandani, Manisha Harish et al.. Evidence-based practice in complementary and alternative medicine : perspectives, protocols, problems, and potential in Ayurveda. Berlin: Springer. ISBN 978-3-642-24564-0. https://books.google.com/books?id=vRQNgXxi5oUC&pg=PA88.
- Gerard, John (1876). Jackson, Benjamin Daydon. ed. A Catalogue of Plants Cultivated in the Garden of John Gerard, in the Years 1596–1599. Cambridge: Cambridge University Press. OCLC 839850873. https://archive.org/details/acatalogueplant00jackgoog.
- Grieve, Maude (1971). A Modern Herbal. 1. New York: Dover Publications Inc. ISBN 978-04862-2-798-6. https://books.google.com/books?id=wwzCAgAAQBAJ&q=grieves+A+Modern+Herbal.
- Kennedy, D.O.; Scholey, Andrew B.; Tindsley, N.T.J.; Perry, E.K.; Wesnes, K.A. (2002). "Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm)". Pharmacology Biochemistry and Behavior 72 (4): 953–964. doi:10.1016/S0091-3057(02)00777-3. ISSN 0091-3057. PMID 12062586. https://www.nature.com/articles/1300230.
- Setzer, William (2009). "Essential Oils and Anxiolytic Aromatherapy". Natural Product Communications 4 (9): 1309. doi:10.1177/1934578X0900400928. ISSN 1555-9475. PMID 19831048.
- Theophrastus (1916). Hort, Arthur F.. ed. Enquiry into Plants and Minor Works on Odours and Weather Signs. 2. London: William Heinemann. OCLC 24148340. https://archive.org/details/enquiryintoplan02theo/page/464/mode/2up.
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, Atanas G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G. et al. (2013). "Ethnopharmacological in vitro studies on Austria's folk medicine-An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs". Journal of Ethnopharmacology 149 (3): 750–771. doi:10.1016/j.jep.2013.06.007. ISSN 1872-7573. PMID 23770053.
- Zirkle, Conway (2001). "Review: Thomas Jefferson's Garden Book. Edwin Morris Betts". Isis 92 (4): 84–85. doi:10.1086/347980. ISSN 1545-6994. https://www.journals.uchicago.edu/doi/10.1086/347980.
| Wikimedia Commons has media related to Melissa officinalis. |
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}} Wikidata ☰ Q148396 entry
 |
