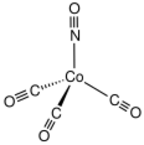
Chemistry:Cobalt tricarbonyl nitrosyl
From HandWiki
| |
| Names | |
|---|---|
| Other names
Cobalt nitrosyl tricarbonyl
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C3CoNO4 | |
| Molar mass | 172.969 g·mol−1 |
| Appearance | red oil |
| Density | 1.47 g/cm3 |
| Melting point | −1.1 °C (30.0 °F; 272.0 K) |
| Boiling point | 50 °C (122 °F; 323 K) |
| Hazards | |
| Main hazards | toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Cobalt tricarbonyl nitrosyl is the organocobalt compound with the formula Co(CO)3(NO). It is a dark red volatile oil that is soluble in nonpolar solvents. The compound is one of the simplest metal nitrosyls. It is highly toxic, reminiscent of the same property for nickel tetracarbonyl.
Synthesis and reactions
Cobalt tricarbonyl nitrosyl is prepared by the treatment of dicobalt octacarbonyl with nitric oxide:[1]
- Co2(CO)8 + 2 NO → 2 Co(CO)3NO + 2 CO
Many other methods have been developed.
The complex undergoes substitution readily by Lewis bases such as tertiary phosphines and isocyanides, concomitant with loss of CO.[2]
References
- ↑ King, R. B. "Organometallic Synthesis, Volume 1: Transition-metal compounds" (1965) Academic Press. ISBN:0124080502
- ↑ Hering, Florian; Berthel, Johannes H. J.; Lubitz, Katharina; Paul, Ursula S. D.; Schneider, Heidi; Härterich, Marcel; Radius, Udo (2016). "Synthesis and Thermal Properties of Novel NHC-Stabilized Cobalt Carbonyl Nitrosyl Complexes". Organometallics 35 (17): 2806–2821. doi:10.1021/acs.organomet.6b00374.
 |

