Chemistry:Copper(I) t-butoxide
From HandWiki
| |
| Names | |
|---|---|
| Other names
copper(1+);2-methylpropan-2-olate
| |
| Identifiers | |
| |
| ChemSpider | |
PubChem CID
|
|
| Properties | |
| C16H36Cu4O4 | |
| Molar mass | 546.644 g·mol−1 |
| Appearance | White solid |
| Density | 1.62 g/cm3 (octamer) |
| Melting point | 260 °C (500 °F; 533 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
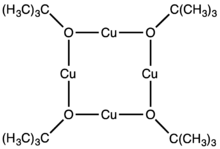
Copper(I) t-butoxide is an alkoxide of copper(I). It is a white sublimable solid. It is a reagent in the synthesis of other copper compounds.[1]
The compound was originally obtained by salt metathesis from lithium tert-butoxide and copper(I) chloride.[2] An octameric form was obtained by alcoholysis of mesitylcopper:[3]
- 8 CuC6H2Me3 + 8 HOBu-t → 8 HC6H2Me3 + [CuOBu-t]8
References
- ↑ Tetsuo Tsuda; Takeshi Takeda; Akira Tsubouchi (2008). "Copper(I) t-Butoxide". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rc212.pub2. ISBN 978-0-471-93623-7.
- ↑ Tsuda, T.; Hashimoto, T.; Saegusa, T. (1972). "Cuprous tert-butoxide. New and useful metalation reagent". Journal of the American Chemical Society 94 (2): 658–659. doi:10.1021/ja00757a069.
- ↑ Håkansson, M.; Lopes, C.; Jagner, S. (2000). "Copper(I) alkoxides: preparation and structural characterisation of triphenylmethoxocopper(I) and of an octanuclear form of t-butoxocopper(I)". Inorganica Chimica Acta 304 (2): 178–183. doi:10.1016/S0020-1693(00)00081-5.
 |

