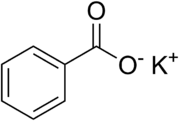
Chemistry:Potassium benzoate
| |||
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Potassium benzoate
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H5KO2 | |||
| Molar mass | 160.213 g·mol−1 | ||
| Appearance | White hygroscopic solid | ||
| Odor | Odorless[1] | ||
| Density | 1.5 g/cm3 | ||
| Melting point | >300 °C (572 °F; 573 K) | ||
| 69.87 g/100 mL (17.5 °C) 73.83 g/100 mL (25 °C) 79 g/100 mL (33.3 °C) 88.33 g/100 mL (50 °C)[2][1] | |||
| Solubility in other solvents | Soluble in ethanol Slightly soluble in methanol Insoluble in ether | ||
| Hazards[3] | |||
| Main hazards | Low toxicity | ||
| GHS pictograms | 
| ||
| GHS Signal word | Warning | ||
| H315, H319 | |||
| P264, P280, P302+352, P305+351+338, P332+313, P337+313, P362 | |||
| NFPA 704 (fire diamond) | |||
| 950 °C (1,740 °F; 1,220 K) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Potassium benzoate (E212), the potassium salt of benzoic acid, is a food preservative that inhibits the growth of mold, yeast and some bacteria. It works best in low-pH products, below 4.5, where it exists as benzoic acid.
Acidic foods and beverages such as fruit juice (citric acid), sparkling drinks (carbonic acid), soft drinks (phosphoric acid), and pickles (vinegar) may be preserved with potassium benzoate. It is approved for use in most countries including Canada , the United States and the European Union, where it is designated by the E number E212.
Potassium benzoate is also used in the whistle in many fireworks.[4]
Synthesis
One very common way to make potassium benzoate is by oxidizing toluene to benzoic acid followed by a neutralization with potassium hydroxide:[5]
Another way to synthesize potassium benzoate in the lab setting is by hydrolyzing methyl benzoate with potassium hydroxide:
Reactions
Potassium benzoate, like sodium benzoate, can be decarboxylated with a strong base and heat:
Mechanism of food preservation
The mechanism of food preservation begins with the absorption of benzoic acid into the cell. If the intracellular pH changes to 5 or lower, the anaerobic fermentation of glucose through phosphofructokinase is decreased by 95%.
Safety and health
Potassium benzoate has low acute toxicity upon oral and dermal exposure.[6] The Food Commission, which campaigns for safer, healthier food in the UK, describes potassium benzoate as "mildly irritant to the skin, eyes and mucous membranes".[7]
Cats have a significantly lower tolerance to benzoic acid and its salts than rats and mice.[8]
Under certain circumstances, such as in the presence of ascorbic acid, benzoate salts can produce benzene in soft drinks. The US Food and Drug Administration states the levels of benzene measured do not pose a safety concern for consumers.[9]
Spectra
Carbon-13 NMR
The carbon-13 NMR shows 5 unique peaks. There are four peaks between 130-140 ppm from the carbon atoms in the benzene ring. There is an additional carbon peak around 178 ppm representing the carbon atom from the carbonyl group.[10]
Infrared spectrum
The following are the main peaks in the IR spectrum.[10]
- 1610: C=O from carbonyl
- 1580: C=C from benzene ring
See also
References
- ↑ 1.0 1.1 "Potassium Benzoate". Emerald Kalama Chemical. http://www.emeraldmaterials.com/cms/kalama/fis_ftp.downloadPublicDoc?p_filename=POTBENZ_TDS_ENG.pdf&p_doc_type=TDS. Retrieved 2014-06-02.
- ↑ Seidell, Atherton; Linke, William F. (1952). Solubilities of Inorganic and Organic Compounds. Van Nostrand. https://books.google.com/books?id=k2e5AAAAIAAJ. Retrieved 2014-05-29.
- ↑ "Potassium benzoate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/23661960#section=GHS-Classification.
- ↑ Press Release from Defense Technical Information Center; article- Potassium Benzoate for Pyrotechnic Whistling Compositions: Its Synthesis and Characterization as an Anhydrous Salt
- ↑ Hills, David J., "Preparation of potassium benzoate", US patent 3867439, published 1975-02-18, assigned to The Dow Chemical Company
- ↑ "Benzoates". United Nations Environment Programme. http://www.inchem.org/documents/sids/sids/benzoates.pdf.
- ↑ [1], The Food Magazine, Issue 77, Food Commission UK
- ↑ Bedford PG, Clarke EG (1972). "Experimental benzoic acid poisoning in the cat". Vet Rec 90 (3): 53–58. doi:10.1136/vr.90.3.53. PMID 4672555.
- ↑ "Questions and Answers on the Occurrence of Benzene in Soft Drinks and Other Beverages". Food and Drug Administration. https://www.fda.gov/Food/FoodborneIllnessContaminants/ChemicalContaminants/ucm055131.hts. "...the levels of benzene found in beverages to date do not pose a safety concern for consumers."
- ↑ 10.0 10.1 SciFinder - Carbon-13 NMR Spectrum for 582-25-2
 |