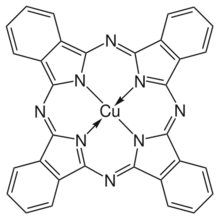
Chemistry:Copper phthalocyanine
| |
| Names | |
|---|---|
| IUPAC name
(29H,31H-phthalocyaninato(2−)-N29,N30,N31,N32)copper(II)
| |
| Other names
Copper(II) phthalocyanine
Monastral blue Phthalocyanine blue Phthalo blue Thalo blue Pigment Blue 15 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C32H16CuN8 | |
| Molar mass | 576.082 g·mol−1 |
| Appearance | dark blue solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Phthalo blue | |
|---|---|
 Phthalocyanine blue pigment powder | |
| Hex triplet | #000F89 |
| Source | The Mother of All HTML Colo(u)r Charts |
| ISCC–NBS descriptor | Vivid blue |
Copper phthalocyanine (CuPc), also called phthalocyanine blue, phthalo blue and many other names, is a bright, crystalline, synthetic blue pigment from the group of phthalocyanine dyes. Its brilliant blue is frequently used in paints and dyes. It is highly valued for its superior properties such as light fastness, tinting strength, covering power and resistance to the effects of alkalis and acids. It has the appearance of a blue powder, insoluble in most solvents including water.
History
The discovery of metal phthalocyanines can be traced to the observation of intensely colored byproducts from reactions of phthalic acid (benzene-1,2-dicarboxylic acid) or its derivatives with sources of nitrogen and metals. CuPc (copper phthalocyanine) was first prepared in 1927 by the reaction of copper(I) cyanide and o-dibromobenzene, which mainly produces colorless phthalonitrile as well as an intensely blue by-product. A couple of years later, workers at Scottish Dyes observed the formation of traces of phthalocyanine dyes in the synthesis of phthalimide by the reaction of phthalic anhydride and ammonia in the presence of metallic iron. In 1937, DuPont started producing copper phthalocyanine blue in the USA under the trade name Monastral Blue after it had been previously launched in Great Britain (ICI) and Germany (I.G. Farbenindustrie) in 1935.[1]
Difficulty was experienced in forming stable dispersions with the first alpha forms, especially in mixtures with rutile titanium, where the blue pigment tended to flocculate. The beta form was more stable, as was the improved stabilized alpha form. Today, there are even more isomeric forms available.
Synonyms and trade names
The substance, IUPAC name (29H,31H-phthalocyaninato(2−)-N29,N30,N31,N32)copper(II), is known by many names[2] such as monastral blue, phthalo blue, helio blue,[3] thalo blue, Winsor blue,[4] phthalocyanine blue, C.I. Pigment Blue 15:2,[5][6] copper phthalocyanine blue,[7] copper tetrabenzoporphyrazine,[8] Cu-phthaloblue,[9] P.B.15.2,[10][11][12] C.I. 74160,[13][14][15] and British Rail Blue.[16] Numerous other trade names and synonyms exist.[17] The abbreviation "CuPc" is also used.[18]
Manufacture
Two manufacturing processes have gained commercial importance for the production of copper phthalocyanine:
- The phthalonitrile process, mainly used in Germany
- The phthalic anhydride/urea process, developed in Great Britain and in the USA.
Both approaches can be carried out either without (baking process) or with a solvent (solvent process). Higher yields may be achieved with the solvent process (> 95%) than with the baking process (70 to 80%), so that the solvent process has initially simulated more interest. However, recents trends show a reverse tendency for the baking process mainly on the grounds of economical and ecological concerns (solvent-free, shorter lead time).
Phthalonitrile process
This approach involves heating phthalonitrile with a copper salt, usually copper(I)chloride at 200°C to 240°C. The gross reaction equation from phthalonitrile may be written as follows:
- [math]\ce{ 4 C6H4(CN)2 + Cu^2+ + 2e- -> CuPc }[/math]
Phthalic anhydride/urea process
The gross reaction equation from phthalic anhydride and urea may be written as follows:
- [math]\ce{ 4 C6H4(CO)2O + 4(NH2)2CO + Cu^2+ + 2e- -> CuPc + 8H2O + 4CO2 + 4NH3 }[/math]
Applications

Catalysis
Metal phthalocyanines have long been examined as catalysts for redox reactions. Areas of interest are the oxygen reduction reaction and the sweetening of gas streams by removal of hydrogen sulfide.[citation needed]
Colorant
Due to its stability, phthalo blue is also used in inks, coatings, and many plastics. The pigment is insoluble and has no tendency to migrate in the material. It is a standard pigment used in printing ink and the packaging industry. Industrial production was of the order of 10,000 tonnes per annum in the 1980s and 1990s in Japan alone.[17] The pigment is the highest volume pigment produced.[19]
All major artists' pigment manufacturers produce variants of copper phthalocyanine, designated color index PB15 (blue) and color indexes PG7 and PG36 (green).
A common component on the artist's palette, phthalo blue is a cool blue with a bias towards green. It has intense tinting strength and easily overpowers the mix when combined with other colors. It is a transparent staining color and can be applied using glazing techniques.
It is present in a wide variety of products,[20] such as color deposition hair conditioner,[21] eye patches, parfum, shampoo, skin-care products, soap, sunscreen, tattoo ink,[22] toothpaste,[23] and even turf colorants. [24]
Research
CuPc has often been investigated in the context of molecular electronics. It is potentially suited for organic solar cells because of its high chemical stability and uniform growth.[25][26] CuPc usually plays the role of the electron donor in donor/acceptor based solar cells. One of the most common donor/acceptor architectures is CuPc/C60 (buckminsterfullerene) which rapidly became a model system for the study of small organic molecules.[27][28] Photon to electron conversion efficiency in such system reaches approximately 5%.
CuPc has also been investigated as a component of organic field-effect transistors.[29] Copper Phthalocyanine (CuPc) has been suggested for data storage in quantum computing, due to the length of time its electrons can remain in superposition.[30] CuPc can be easily processed into a thin film for use in device fabrication, which makes it an attractive qubit candidate.[31]
Approximately 25% of all artificial organic pigments are phthalocyanine derivatives.[32] Copper phthalocyanine dyes are produced by introducing solubilizing groups, such as one or more sulfonic acid functions. These dyes find extensive use in various areas of textile dyeing (Direct dyes for cotton), for spin dyeing and in the paper industry. Direct blue 86 is the sodium salt of CuPc-sulfonic acid, whereas direct blue 199 is the quaternary ammonium salt of the CuPc-sulfonic acid. The quaternary ammonium salts of these sulfonic acids are used as solvent dyes because of their solubility in organic solvents, such as Solvent Blue 38 and Solvent Blue 48. The dye derived from cobalt phthalocyanine and an amine is Phthalogen Dye IBN. 1,3-Diiminoisoindolene, the intermediate formed during phthalocyanine manufacture, used in combination with a copper salt affords the dye GK 161.
Copper phthalocyanine is also used as a source material for manufacture of Phthalocyanine Green G.
Other related and commercially available phthalocyanines blue pigments are:
- Pigment Blue 16 – metal-free phthalocyanine
- Pigment Blue 75 – cobalt phthalocyanine
- Pigment Blue 79 – aluminum phthalocyanine
Structure, reactivity and properties

Copper phthalocyanine is a complex of copper(II) with the conjugate base of phthalocyanine, i.e. Cu2+Pc2−. The description is analogous to that for copper porphyrins, which are also formally derived by double deprotonation of porphyrins. CuPc belongs to the D4h point group. It is paramagnetic with one unpaired electron per molecule.
The substance is practically insoluble in water (< 0.1 g/100 ml at 20 °C (68 °F)),[34] but soluble in concentrated sulfuric acid.[17] Density of the solid is ~1.6 g/cm3.[17] The color is due to a π–π* electronic transition, with λmax ≈ 610 nm.[35]
Crystalline phases
CuPc crystallizes in various forms (polymorphs). Five different polymorphs have been identified:[36][37][38][39] phases α, β, η, γ and χ. The two most common structures in CuPc are the β phase and the metastable α phase. Those phases can be distinguished by the overlap of their neighboring molecules. The α phase has a larger overlap and thus, a smaller Cu-Cu spacing (~3.8 Å) compared to the β phase (~4.8 Å).[40]
Toxicity and hazards
The compound is non-biodegradable, but not toxic to fish or plants.[17] No specific dangers have been associated with this compound.[41] Oral LD50 in mammals is estimated to be greater than 5 g per kg, with no ill effects found at that level of ingestion,[17] for chronic ingestion estimated dose of low concern was 0.2 mg/kg per day in rats.[17] No evidence indicates carcinogenic effects.[17] Sulfonated phthalocyanine has been found to cause neuroanatomical defects in developing chicken embryos when injected directly into incubating eggs.[42]
See also
- Phthalocyanine Green G
- British Rail corporate liveries § Rail Blue – use of the pigment as the standard livery for British Rail trains from 1965 onwards.
- The Joy of Painting – oil paint based on the pigment was frequently used on the show.
- List of colors
References
- ↑ Löbbert, Gerd (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a20_213..
- ↑ "Substance Information". https://echa.europa.eu/substance-information/-/substanceinfo/100.005.169.
- ↑ Toxic Substances Control Act Chemical Substance Inventory: volume 2
- ↑ Spectroscopic Properties of Inorganic and Organometallic Compounds: volume 40
- ↑ Chem Product Index by Friedrich W. Derz
- ↑ Coloring of Plastics: Fundamentals, r. Robert A. Charvat
- ↑ Paint and Coating Testing Manual, e. Joseph V. Koleske
- ↑ User guide and indices to the initial inventory, substance name index, US EPA
- ↑ Industrial Organic Pigments: Production, Crystal Structures, Properties, Applications by Klaus Hunger & Martin U. Schmidt
- ↑ The Porphyrin Handbook: Applications of Phthalocyanines, e. Karl Kadish, Kevin M. Smith & Roger Guilard
- ↑ Tattoo Inks: Analysis, Pigments, Legislation by Gerald Prior
- ↑ Pigment + Füllstoff: Tabellen by Olaf Lückert
- ↑ Material Safety Data Sheets Service 7:89, Information Handling Services
- ↑ Coloring of Food, Drugs, and Cosmetics by Gisbert Otterstätter
- ↑ Chemical Formulation: An Overview of Surfactant Based Chemical Preparations Used in Everyday Life by Anthony E. Hargreaves
- ↑ Waterloo Station: A History of London's busiest terminus by Robert Lordan
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 17.6 17.7 COPPER PHTHALOCYANINE, CAS No.: 147-14-8 inchem.org
- ↑ e.g. Structural and Transport Properties of Copper Phthalocyanine (CuPc) Thin Films www.egmrs.org
- ↑ Gregory, Peter (2000). "Industrial applications of phthalocyanines". Journal of Porphyrins and Phthalocyanines (worldscinet.com) 4 (4): 432–437. doi:10.1002/(SICI)1099-1409(200006/07)4:4<432::AID-JPP254>3.0.CO;2-N. http://www.worldscinet.com/jpp/04/0404/S1088424600000669.html.
- ↑ "Ci 74160 (With Product List)". https://incidecoder.com/ingredients/ci-74160.
- ↑ "Color Deposition Conditioner "Ultra Violet"". https://kosterkeunen.com/formulas/color-deposition-conditioner-ultra-violet#post-5632.
- ↑ Miranda, Michelle D. (2016) Forensic Analysis of Tattoos and Tattoo Inks. Routledge. ISBN:9780367778439 p. 163: Muddy Water Blue
- ↑ "Dentalux Complex 7 Total Care Plus Zahncreme Inhaltsstoffe – Hautschutzengel". https://hautschutzengel.de/_/produkt/51660.html#h2tab1a.
- ↑ "Vertmax Turf pigment and paint". 17 February 2022. https://gilbasolutions.com/guide-to-turf-pigment-and-paint-7.
- ↑ Szybowicz, M (October 2004). "High temperature study of FT-IR and Raman scattering spectra of vacuum deposited CuPc thin films". Journal of Molecular Structure 704 (1–3): 107–113. doi:10.1016/j.molstruc.2004.01.053. Bibcode: 2004JMoSt.704..107S.
- ↑ Bala, M; Wojdyla, M; Rebarz, M; Szybowic, M; Drozdowski, M; Grodzicki, A; Piszczek, P (2009). "Influence of central metal atom in MPc (M = Cu, Zn, Mg, Co) on Raman, FT-IR, absorbance, reflectance, and photoluminescence spectra". J. Optoelectron. Adv. M. 11 (3): 264–269. https://www.researchgate.net/publication/255698291.
- ↑ Jailaubekov, Askat E.; Willard, Adam P.; Tritsch, John R.; Chan, Wai-Lun; Sai, Na; Gearba, Raluca; Kaake, Loren G.; Williams, Kenrick J. et al. (2013). "Hot charge-transfer excitons set the time limit for charge separation at donor/acceptor interfaces in organic photovoltaics". Nature Materials 12 (1): 66–73. doi:10.1038/nmat3500. PMID 23223125. Bibcode: 2013NatMa..12...66J.
- ↑ Xin, Li (January 2013). "CuPc/C60 bulk heterojunction photovoltaic cells with evidence of phase segregation". Organic Electronics 14: 250–254. doi:10.1016/j.orgel.2012.10.041.
- ↑ Chaidogiannos, G.; Petraki, F.; Glezos, N.; Kennou, S.; Nešpůrek, S. (2009). "Low voltage operating OFETs based on solution-processed metal phthalocyanines". Applied Physics A 96 (3): 763. doi:10.1007/s00339-009-5268-1. Bibcode: 2009ApPhA..96..763C.
- ↑ New material for quantum computing discovered out of the blue. phys.org. October 27, 2013
- ↑ Quenqua, Douglas (November 4, 2013). "A Key to Quantum Computing, Close to Home". The New York Times. https://www.nytimes.com/2013/11/05/science/a-key-to-quantum-computing-close-to-home.html.
- ↑ Gerd Löbbert "Phthalocyanines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a20_213.
- ↑ Erk, Peter; Hengelsberg, Heidi; Haddow, Mairi F.; Van Gelder, Richard (2004). "The innovative momentum of crystal engineering". CrystEngComm 6 (78): 474. doi:10.1039/b409282a.
- ↑ Copper phthalocyanine chemblink.com
- ↑ Rzepa, H. S. Monastral: the colour of blue. .imperial.ac.uk )
- ↑ James H., Sharp; Martin, Abkowitz (1973). "Dimeric Structure of a Copper Phthalocyanine Polymorph". J. Phys. Chem. 77 (11): 477–481. doi:10.1021/j100623a012.
- ↑ Jacques M., Assour (1965). "On the Polymorphic Modifications of Phthalocyanines". J. Phys. Chem. 69 (7): 2295–2299. doi:10.1021/j100891a026.
- ↑ Hassan, A. K.; Gould, R. D. (2006). "Structural Studies of Thermally Evaporated Thin Films of Copper Phthalocyanine". Physica Status Solidi A 132 (1): 91–101. doi:10.1002/pssa.2211320110. Bibcode: 1992PSSAR.132...91H.
- ↑ Hai, Wang; Soumaya, Mauthoor; Salahud, Din; Jules A., Gardener; Rio, Chang; Marc, Warner; Gabriel, Aeppli; David W., McComb et al. (June 7, 2010). "Ultralong Copper Phthalocyanine Nanowires with New Crystal Structure and Broad Optical Absorption". ACS Nano 4 (7): 3921–3926. doi:10.1021/nn100782w. PMID 20527798.
- ↑ Amy C, Cruickshank; Christian J, Dotzler; Salahud, Din; Sandrine, Heutz; Michael F, Toney; Mary P, Ryan (2012). "The crystalline structure of copper phthalocyanine films on ZnO(1100)". Journal of the American Chemical Society 134 (35): 14302–14305. doi:10.1021/ja305760b. PMID 22897507.
- ↑ Safety data sheet cornelius.co.uk
- ↑ Sandor, S; Prelipceanu, O; Checiu, I (1985). "Sulphonated phthalocyanine induced caudal malformative syndrome in the chick embryo.". Morphol Embryol (Bucur) 31 (3): 173–81. PMID 2931590.
External links
- Discovery of a new pigment – "Monastral blue"[Usurped!] colorantshistory.org
- Patrick Linstead talking about phthalocyanine Imperial College London, Chemistry department
 |

