Chemistry:Cyclobutadiene
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclobuta-1,3-diene | |||
| Other names
1,3-Cyclobutadiene
Cyclobutadiene [4]Annulene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C4H4 | |||
| Molar mass | 52.076 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Cyclobutadiene is an organic compound with the formula C
4H
4. It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure.[1][2]
Structure and reactivity
The compound is the prototypical antiaromatic hydrocarbon with 4 pi electrons (or π electrons). It is the smallest [n]-annulene ([4]-annulene). Its rectangular structure is the result of a pseudo[3]- (or second order) Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state.[4] The electronic states of cyclobutadiene have been explored with a variety of computational methods.[5] The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence isomers. This distortion indicates that the pi electrons are localized, in agreement with Hückel's rule which predicts that a π-system of 4 electrons is not aromatic.
In principle, another situation is possible. Namely, cyclobutadiene could assume an undistorted square geometry, if it adopts a triplet spin state. While a theoretical possibility, the triplet form of the parent cyclobutadiene and its substituted derivatives remained elusive for decades. However, in 2017, the square triplet excited state of 1,2,3,4-tetrakis(trimethylsilyl)-1,3-cyclobutadiene was observed spectroscopically, and a singlet-triplet gap of EST = 13.9 kcal/mol (or 0.6 eV per molecule) was measured for this compound.[6]
Synthesis
Several cyclobutadiene derivatives have been isolated with steric bulky substituents. Orange tetrakis (tert-butyl)cyclobutadiene arises by thermolysis of its isomer tetra-tert-butyltetrahedrane. Although the cyclobutadiene derivative is stable (with respect to dimerization), it decomposes upon contact with O
2.[7][8]
Trapping
Samples of cyclobutadiene are unstable since the compound dimerizes at temperatures above 35 K by a Diels-Alder reaction.[9] By suppressing bimolecular decomposition pathways, cyclobutadiene is well-behaved. Thus it has been generated in a hemicarceplex.[2] The inclusion compound is generated by photodecarboxylation of bicyclopyran-2-one.[10] When released from the host–guest complex, cyclobutadiene dimerizes and then converts to cyclooctatetraene.
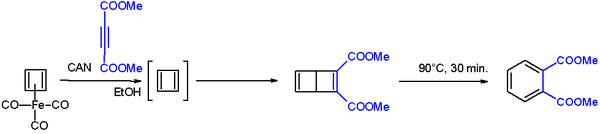
After numerous attempts, cyclobutadiene was first generated by oxidative degradation of cyclobutadieneiron tricarbonyl with ammonium cerium(IV) nitrate.[11][12] When liberated from the iron complex, cyclobutadiene reacts with electron-deficient alkynes to form a Dewar benzene:[13]
The Dewar benzene converts to dimethyl phthalate on heating at 90 °C.
One cyclobutadiene derivative is also accessible through a [2+2]cycloaddition of a di-alkyne. In this particular reaction the trapping reagent is 2,3,4,5-tetraphenylcyclopenta-2,4-dienone and one of the final products (after expulsion of carbon monoxide) is a cyclooctatetraene:[14]
See also
References
- ↑ Kollmar, H.; Staemmler, V. (1977). "A theoretical study of the structure of cyclobutadiene H. Kollmar, V. Staemmler; J. Am. Chem. Soc.". Journal of the American Chemical Society 99 (11): 3583–3587. doi:10.1021/ja00453a009.
- ↑ 2.0 2.1 Cram, Donald J.; Tanner, Martin E.; Thomas, Robert (1991). "The Taming of Cyclobutadiene Donald J. Cram, Martin E. Tanner, Robert Thomas". Angewandte Chemie International Edition in English 30 (8): 1024–1027. doi:10.1002/anie.199110241.
- ↑ Albright, Burdett and Whangbo, Orbital Interactions in Chemistry 2nd ed. pp 282ff.
- ↑ Peter Senn (1992). "A Simple Quantum Mechanical Model that Illustrates the Jahn-Teller Effect". J. Chem. Educ. 69 (10): 819. doi:10.1021/ed069p819. Bibcode: 1992JChEd..69..819S.
- ↑ Balkova, A.; Bartlett, R. J. J. Chem. Phys. 1994, 101, 8972–8987.
- ↑ Kostenko, Arseni; Tumanskii, Boris; Kobayashi, Yuzuru; Nakamoto, Masaaki; Sekiguchi, Akira; Apeloig, Yitzhak (2017-07-03). "Spectroscopic Observation of the Triplet Diradical State of a Cyclobutadiene" (in en). Angewandte Chemie International Edition 56 (34): 10183–10187. doi:10.1002/anie.201705228. ISSN 1433-7851. PMID 28635054.
- ↑ Günther Maier; Stephan Pfriem; Ulrich Schäfer; Rudolf Matusch (1978). "Tetra-tert-butyltetrahedrane". Angew. Chem. Int. Ed. Engl. 17 (7): 520. doi:10.1002/anie.197805201.
- ↑ Hermann Irngartinger; Norbert Riegler; Klaus-Dieter Malsch; Klaus-Albert Schneider; Günther Maier (1980). "Structure of Tetra-tert-butylcyclobutadiene". Angewandte Chemie International Edition in English 19 (3): 211–212. doi:10.1002/anie.198002111.
- ↑ Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry: Part A: Structure and Mechanisms (5th ed.). Springer. p. 725. ISBN 978-0-387-44897-8. https://books.google.com/books?id=g5dYyJMBhCoC&q=cyclobutadiene+dimerization+Diels-Alder&pg=PA725.
- ↑ E. J. Corey, Jacques Streith (1964). "Internal Photoaddtion Reactions of 2-Pyrone and N-Methyl-2-pyridone: A New Synthetic Approach to Cyclobutadiene". J. Am. Chem. Soc. 86 (5): 950–951. doi:10.1021/ja01059a059.
- ↑ G. F. Emerson; L. Watts; R. Pettit (1965). "Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes". J. Am. Chem. Soc. 87: 131–133. doi:10.1021/ja01079a032.
- ↑ R. Pettit; J. Henery (1970). "Cyclobutadieneiron tricarbonyl". Organic Syntheses 50: 21. doi:10.15227/orgsyn.050.0021.
- ↑ L. Watts; J. D. Fitzpatrick; R. Pettit (1965). "Cyclobutadiene". J. Am. Chem. Soc. 87 (14): 3253–3254. doi:10.1021/ja01092a049.
- ↑ Chung-Chieh Lee; Man-kit Leung; Gene-Hsiang Lee; Yi-Hung Liu; Shie-Ming Peng (2006). "Revisit of the Dessy-White Intramolecular Acetylene-Acetylene [2 + 2 Cycloadditions"]. J. Org. Chem. 71 (22): 8417–8423. doi:10.1021/jo061334v. PMID 17064014. http://ntur.lib.ntu.edu.tw/bitstream/246246/164494/1/30.pdf.
 |

![Acetylene-Acetylene [2 + 2] Cycloadditions Chung-Chieh Lee 2006](/wiki/images/thumb/c/c8/CyclobutadienSynthDessyWhite.png/400px-CyclobutadienSynthDessyWhite.png)
