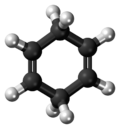
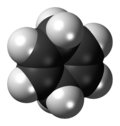
Chemistry:Cyclohexa-1,4-diene
| |||
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohexa-1,4-diene[1] | |||
| Other names
1,4-Cyclohexadiene[citation needed]
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | 1,4-CHDN | ||
| 1900733 | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| 1656 | |||
| MeSH | 1,4-cyclohexadiene | ||
PubChem CID
|
|||
| UNII | |||
| UN number | 3295 | ||
| |||
| |||
| Properties | |||
| C6H8 | |||
| Molar mass | 80.130 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 0.847 g cm−3 | ||
| Melting point | −50 °C; −58 °F; 223 K | ||
| Boiling point | 82 °C; 179 °F; 355 K | ||
| -48.7·10−6 cm3/mol | |||
Refractive index (nD)
|
1.472 | ||
| Thermochemistry | |||
Heat capacity (C)
|
142.2 J K−1 mol−1 | ||
Std molar
entropy (S |
189.37 J K−1 mol−1 | ||
Std enthalpy of
formation (ΔfH⦵298) |
63.0-69.2 kJ mol−1 | ||
Std enthalpy of
combustion (ΔcH⦵298) |
-3573.5--3567.5 kJ mol−1 | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS Signal word | DANGER | ||
| H225, H340, H350, H373 | |||
| P201, P210, P308+313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −7 °C (19 °F; 266 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,4-Cyclohexadiene is an organic compound with the formula C6H8. It is a colourless, flammable liquid that is of academic interest as a prototype of a large class of related compounds called terpenoids, an example being γ-terpinene. An isomer of this compound is 1,3-cyclohexadiene.
Synthesis and reactions
In the laboratory, substituted 1,4-cyclohexadienes are synthesized by Birch reduction of related aromatic compounds using an alkali metal dissolved in liquid ammonia and a proton donor such as an alcohol. In this way, over reduction to the fully saturated ring is avoided.
1,4-Cyclohexadiene and its derivatives are easily aromatized, the driving force being the formation of an aromatic ring. The conversion to an aromatic system may be used to trigger other reactions, such as the Bergman cyclization.[2]
References
- ↑ "1,4-cyclohexadiene - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related Records. https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=12343&loc=ec_rcs. Retrieved 12 October 2011.
- ↑ John C. Walton, Fernando Portela-Cubillo "1,4-Cyclohexadiene" Encyclopedia of Reagents for Organic Synthesis 2007 John Wiley & Sons. doi:10.1002/047084289X.rn00806
External links
- The photochemistry of 1,4-cyclohexadiene in solution and in the gas phase
- NIST Chemistry WebBook Reaction thermochemistry data
 |