Chemistry:Decacarbonyldihydridotriosmium
| |
| Names | |
|---|---|
| IUPAC names
Decacarbonyldihydridotriosmium,
Decacarbonyl-1κ3C,2κ3C,3κ4C- | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| H2Os3(CO)10 | |
| Molar mass | 852.81 g/mol |
| Appearance | Deep purple-violet crystals |
| Density | 3.48 g/cm3 |
| Boiling point | decomposes |
| insoluble | |
| Solubility in other solvents | reacts with Chlorocarbons |
| Structure | |
| triangular cluster | |
| Hazards | |
| Main hazards | Toxic |
| Related compounds | |
Related compounds
|
Os3(CO)12 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
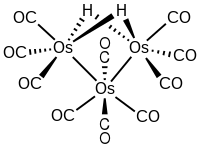
Decacarbonyldihydridotriosmium is an organoosmium compound with the formula H2Os3(CO)10. This purple-violet crystalline air-stable cluster is noteworthy because it is electron-deficient and hence adds a variety of substrates.
Structure and synthesis
The trinuclear cluster features an isosceles triangular array of metals with one short edge (rOs-Os = 2.68 Å), which is spanned by the two hydride ligands, and two longer edges (rOs-Os = 2.81 Å).[1] It can be described as Os(CO)4[Os(CO)3(μ-H)]2. The bonding in the Os2H2 subunit has been compared to the 3-center, 2e bonding in diborane. The molecule forms a variety of adducts with loss of H2.[2]
It is prepared by purging a solution of Os3(CO)12 in octane (or other inert solvent of similar boiling point) with H2.[3]
- Os3(CO)12 + H2 → Os3H2(CO)10 + 2 CO
Reactions
The cluster reacts with a wide range of reagents under mild conditions. Illustrative is its reaction with diazomethane to give Os3(CO)10(μ-H)(μ-CH3), exhibiting an agostic interaction, the first identified in a metal cluster.[4][5]
References
- ↑ Melvyn Rowen Churchill; Frederick J. Hollander; John P. Hutchinson (1977). "Structural studies on polynuclear osmium carbonyl hydrides. 5.Crystal structure and molecular geometry of di-μ-hydrido-decacarbonyltriosmium, (μ-H)2Os3(CO)10". Inorg. Chem. 28 (11): 2697–2700. doi:10.1021/ic50177a006.
- ↑ Keister, J. B.; Shapley, J. R. "Solution Structures and Dynamics of complexes of Decacarbonyldihydrotriosmium with Lewis Bases" Inorganic Chemistry 1982, volume 21, pages 3304–3310; doi:10.1021/ic00139a011.
- ↑ Kaesz, H. D. (1990). "Decacarbonyldi-μ-Hydridotriosmium: Os3(μ-H)2(CO)10". Inorganic Syntheses 28: 238–39. doi:10.1002/9780470132593.ch60.
- ↑ Calvert, R. Bruce; Shapley, John R. (1977). "Activation of Hydrocarbons by Unsaturated metal Cluster Complexes. 6. Synthesis and Characterization of Methyldecacarbonylhydridotriosmium, Methylenedecacarbonyldihydridotriosmium, and Methylidynenonacarbonyltrihydridotriosmium. Interconversion of Cluster-Bound Methyl and Methylene Ligands". Journal of the American Chemical Society 99 (15): 5225–5226. doi:10.1021/ja00457a077.
- ↑ Calvert, R. Bruce; Shapley, John R. (1978). "Decacarbonyl(methyl)hydrotriosmium: NMR Evidence for a Carbon...Hydrogen...Osmium Interaction". Journal of the American Chemical Society 100 (24): 7726–7727. doi:10.1021/ja00492a047.
 |

