Chemistry:Diethylphosphite
| |
| Names | |
|---|---|
| Preferred IUPAC name
Diethyl phosphonate | |
| Other names
diethyl phosphonite; DEP; Phosphonic acid, diethyl ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| 4-01-00-01329 | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H11O3P | |
| Molar mass | 138.103 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.072 g/cm3 |
| Boiling point | 50-51 °C at 2 mm Hg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
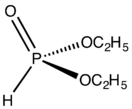
Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid.[1] The molecule is tetrahedral.
Synthesis and properties
The compound was probably prepared in the 1850s by combining phosphorus trichloride and ethanol, but intentional preparations came later. It arises as follows:[2]
- PCl3 + 3 C2H5OH → (C2H5O)2P(O)H + 2 HCl + C2H5Cl
Under similar conditions but in the presence of base, triethyl phosphite results:[3]
- PCl3 + 3 EtOH + 3 R3N → P(OEt)3 + 3 R3NH + 3 Cl−
Many analogues of diethyl phosphite can be prepared.[4][5] Despite being named as a phosphite the compound exists overwhelmingly in its phosphonate form, (C2H5O)2P(O)H, a property it shares with its parent acid phosphorous acid. Nonetheless many of its reactions appear to proceed via the minor phosphorus(III) tautomer.[6]
- (C2H5O)2PIII(OH) ⇌ (C2H5O)2PV(O)H, K = 15 x 106 (25°C, aqueous)[7]
Reactions
Hydrolysis and alcoholysis
Diethyl phosphite hydrolyzes to give phosphorous acid. Hydrogen chloride accelerates this conversion.:[2]
Diethyl phosphite undergoes transesterification upon treating with an alcohol. For alcohols of high boiling points, the conversion can be driven by removal of ethanol:[8]
- (C2H5O)2P(O)H + 2 ROH → (RO)2P(O)H + 2 C2H5OH
Similarly amines can displace ethoxide:[9]
- (C2H5O)2P(O)H + RNH2 → (C2H5O)(RN(H)P(O)H + C2H5OH
P-alkylation
Diethyl phosphite undergoes deprotonation with potassium tert-butoxide. This reactivity allows alkylation at phosphorus (Michaelis–Becker reaction):[10]
- (C2H5O)2P(O)H + KOtBu → (C2H5O)2P(O)K + HOtBu
- (C2H5O)2P(O)K + RBr → (C2H5O)2P(O)R + KBr
For converting aryl halides, palladium-catalysis can be employed.[1] The C-P coupling process is reminiscent of the Buchwald-Hartwig amination.
Reaction of diethyl phosphite with Grignard reagents results in initial deprotonation followed by displacement of the ethoxy groups.[11][12] This reactivity provides a route to secondary phosphine oxides, such as dimethylphosphine oxide as shown in the following pair of idealized equations:
- (C2H5O)2P(O)H + CH3MgBr → (C2H5O)2P(O)MgBr + CH4
- (C2H5O)2P(O)MgBr + 2 CH3MgBr → (CH3)2P(O)MgBr + 2 MgBr(OC2H5)
- (CH3)2P(O)MgBr + H2O → (CH3)2P(O)H + MgBr(OH)
Hydrophosphonylation
Diethyl phosphite can add across unsaturated groups via a hydrophosphonylation reaction. For example, it adds to aldehydes in a manner similar to the Abramov reaction:
- (C2H5O)2P(O)H + RCHO → (C2H5O)2P(O)CH(OH)R
It can also add to imines in the Pudovik reaction and Kabachnik–Fields reaction,[13] in both cases forming aminophosphonates
See also
References
- ↑ 1.0 1.1 Green, Kenneth (2001). Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rd211. ISBN 0471936235.
- ↑ 2.0 2.1 Malowan, J. E. (1953). "Diethyl Phosphite". Inorganic Syntheses. 4. 58–60. doi:10.1002/9780470132357.ch19. ISBN 9780470132357.
- ↑ Ford-Moore, A. H.; Perry, B. J. (1951). "Triethyl Phosphite". Org. Synth. 31: 111. doi:10.15227/orgsyn.031.0111.
- ↑ Pedrosa, Leandro (March 20, 2011). "Esterification of Phosphorus Trichloride with Alcohols; Diisopropyl phosphonate". ChemSpider Synthetic Pages (Royal Society of Chemistry): SyntheticPage 488. doi:10.1039/SP488. http://cssp.chemspider.com/488. Retrieved July 10, 2017.
- ↑ Fakhraian, H.; Mirzaei, A. (2004). "Reconsideration of the Base-Free Batch-Wise Esterification of Phosphorus Trichloride with Alcohols". Org. Process Res. Dev. 8 (3): 401–404. doi:10.1021/op049958v.
- ↑ Doak, G. O.; Freedman, Leon D. (1961). "The Structure and Properties of the Dialkyl Phosphonates". Chem. Rev. 61 (1): 31–44. doi:10.1021/cr60209a002.
- ↑ Guthrie, J. Peter (1979). "Tautomerization Equilibria for Phosphorous Acid and its Ethyl Esters, Free Energies of Formation of Phosphorous and Phosphonic Acids and their Ethyl Esters, and p Ka Values for Ionization of the P—H Bond in Phosphonic Acid and Phosphonic Esters". Canadian Journal of Chemistry 57 (2): 236–239. doi:10.1139/v79-039.
- ↑ Malowan, John E. (1953). "Dioctyl Phosphite". Inorganic Syntheses. 4. 61–62. doi:10.1002/9780470132357.ch20. ISBN 9780470132357.
- ↑ John M. Read, Yu-Pu Wang, Rick L. Danheiser (2015). "Synthesis of Phosphoryl Ynamides by Copper-Catalyzed Alkynylation of Phosphoramidates. Preparation of Diethyl Benzyl(oct-1-yn-1-yl)phosphoramidate". Org. Synth. 92: 156. doi:10.15227/orgsyn.092.0156.
- ↑ Boeckman, Robert K.; Perni, Robert B.; Macdonald, James E.; Thomas, Anthony J. (1988). "6-Diethylphosphonomethyl-2,2-dimethyl-1,3-dioxen-4-one (Phosphonic acid, [(2,2-dimethyl-4-oxo-4H-1,3-dioxin-6-yl)methyl-, diethyl ester)"]. Organic Syntheses 66: 194. doi:10.15227/orgsyn.066.0194. http://www.orgsyn.org/demo.aspx?prep=CV8P0192.; Collective Volume, 8, pp. 192
- ↑ Hays, Hugh R. (1968). "Reaction of diethyl phosphonate with methyl and ethyl Grignard reagents". J. Org. Chem. 33 (10): 3690–3694. doi:10.1021/jo01274a003.
- ↑ Busacca, Carl A.; Lorenz, Jon C.; Sabila, Paul; Haddad, Nizar; Senanyake, Chris H. (2007). "Synthesis of Electron-Deficient Secondary Phosphine Oxides and Secondary Phosphines: Bis[3,5-bis(trifluoromethyl)phenylphosphine Oxide and Bis[3,5-bis(trifluoromethyl)phenyl]phosphine"]. Organic Syntheses 84: 242. doi:10.15227/orgsyn.084.0242. http://www.orgsyn.org/demo.aspx?prep=v84p0242.
- ↑ Keglevich, György; Bálint, Erika (1 November 2012). "The Kabachnik–Fields Reaction: Mechanism and Synthetic Use". Molecules 17 (11): 12821–12835. doi:10.3390/molecules171112821. PMID 23117425.

 |
