Chemistry:Dimethyl dithiophosphoric acid
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
O,O-Dimethyl phosphorodithioate | |
| Other names
O,O-Dimethyl dithiophosphoric acid; Dimethyl dithiophosphate; Dimethyl phosphorodithioate; Dimethyl ester of phosphorodithioic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H7O2PS2 | |
| Molar mass | 158.17 g·mol−1 |
| Appearance | colorless liquid |
| Boiling point | 62–64 °C (144–147 °F; 335–337 K) 0.5 mm Hg |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H226, H242, H290, H302, H314, H332, H361, H412 | |
| P201, P202, P210, P233, P234, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P281, P301+312, P301+330+331, P303+361+353, P304+312, P304+340, P305+351+338, P308+313, P310 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
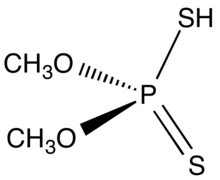
Dimethyl dithiophosphoric acid is the organophosphorus compound with the formula (CH3O)2PS2H. It is the processor for production of the organothiophosphate insecticide Malathion. Although samples can appear dark, the compound is a colorless, distillable liquid.[1]
It is prepared by treating phosphorus pentasulfide with methanol:[2]
- P2S5 + 4 CH3OH → 2 (CH3O)2PS2H + H2S
See also
References
- ↑ J. Svara, N. Weferling, T. Hofmann "Phosphorus Compounds, Organic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2006. doi:10.1002/14356007.a19_545.pub2
- ↑ Lefferts, J. L.; Molloy, K. C.; Zuckerman, J. J.; Haiduc, I.; Guta, C.; Ruse, D., "Oxy and thio phosphorus acid derivatives of tin. 1. Triorganotin(IV) dithiophosphate esters", Inorganic Chemistry 1980, volume 19, 1662-1670. doi:10.1021/ic50208a046
 |


