Chemistry:Disodium glutamate
From HandWiki
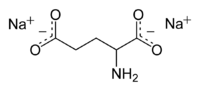
| |
| Names | |
|---|---|
| IUPAC name
Disodium 2-aminopentanedioate
| |
| Other names
DSG
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H7NNa2O4 | |
| Molar mass | 191.09 g/mol |
| Appearance | white crystalline powder |
| Odor | practically odorless |
| Boiling point | 225 °C (437 °F; 498 K) (decomposes) |
| 73.9 g/100 mL (25 °C) | |
| Solubility | sparingly soluble in alcohol |
| Acidity (pKa) | 6.8 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
16600 mg.mg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Disodium glutamate, abbreviated DSG, (Na2C5H7NO4) is a sodium salt of glutamic acid.[1] It is used as a flavoring agent to impart umami flavor.
Formation
Disodium glutamate can be produced by neutralizing glutamic acid with two molar equivalents of sodium hydroxide (NaOH).
See also
References
 |
