Chemistry:Ecdysone
| |
| |
| Names | |
|---|---|
| IUPAC name
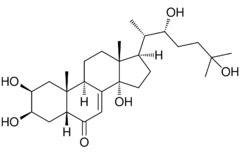
(22R)-2β,3β,14α,22,25-Pentahydroxy-5β-cholest-7-en-6-one
| |
| Systematic IUPAC name
(1R,3aS,5aR,7R,8S,9aR,9bR,11aR)-1-[(2S,3R)-3,6-Dihydroxy-6-methylheptan-2-yl]-3a,7,8-trihydroxy-9a,11a-dimethyl-1,2,3,3a,5a,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-5H-cyclopenta[a]phenanthren-5-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H44O6 | |
| Molar mass | 464.63 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ecdysone is a prohormone of the major insect molting hormone 20-hydroxyecdysone, secreted from the prothoracic glands. It is of steroidal structure. Insect molting hormones (ecdysone and its homologues) are generally called ecdysteroids. Ecdysteroids act as moulting hormones of arthropods but also occur in other related phyla where they can play different roles.[1][2] In Drosophila melanogaster, an increase in ecdysone concentration induces the expression of genes coding for proteins that the larva requires. It causes chromosome puffs (sites of high expression) to form in polytene chromosomes. Recent findings in the laboratory of Chris Q. Doe have found a novel role of this hormone in regulating temporal gene transitions within neural stem cells of the fruit fly.[3] Ecdysone and other ecdysteroids also appear in many plants mostly as a protection agent (toxins or antifeedants) against herbivorous insects.[4] These phytoecdysteroids have been reputed to have medicinal value. They are part of herbal adaptogenic remedies like Cordyceps, yet an ecdysteroid precursor in plants has been shown to have cytotoxic properties[5] as well as antioxidant properties on lipid peroxidation.[6]
Tebufenozide, sold under the Bayer trademark MIMIC,[7] has ecdysteroid activity although its chemical structure has little resemblance to the ecdysteroids.[citation needed]
See also
- Ecdysone receptor
- PTTH - Metamorphosis Initiator hormone
External links
- Ecdybase, The Ecdysone Handbook - a free online ecdysteroids database
Notes
- ↑ Hoffmeister, Hans; Rufer, Clemens; Ammon, Helmut (February 1, 1965). "Ausscheidung von Ecdyson bei Insekten". Zeitschrift für Naturforschung B: Chemie, Biochemie, Biophysik, Biologie und verwandte Gebiete 20 (2): 130–133. doi:10.1515/znb-1965-0207. Physiologisch-Chemisches Institut der Universität Marburg/Lahn. ISSN 0044-3174. PMID 14345159.
- ↑ KARLSON P, HOFFMEISTER H: On the biogenesis of ecdyson. Conversion of cholesterol into ecdyson., Hoppe Seylers Z Physiol Chem. 1963 Mar;331:289-300. German. PMID 1396254
- ↑ Syed, Mubarak Hussain; Mark, Brandon; Doe, Chris Q. (2017). "Steroid hormone induction of temporal gene expression in Drosophila brain neuroblasts generates neuronal and glial diversity". eLife 6. doi:10.7554/eLife.26287. PMID 28394252.
- ↑ "On the distribution of phytoecdysteroids in plants". Cellular and Molecular Life Sciences 58 (8): 1121–1132. 2001. doi:10.1007/PL00000926. PMID 11529504.
- ↑ Wang YS, Yang JH, Luo SD, Zhang HB, Li L, Molecules. 2007;12(3):536-42
- ↑ "New functions of 20-hydroxyecdysone in lipid peroxidation". Journal of Oleo Science. 50 (6): 497–506. 2001. doi:10.5650/jos.50.497. https://www.jstage.jst.go.jp/article/jos/50/6/50_6_497/_pdf.
- ↑ apvma.gov.au: "Tebufenozide in the product Mimic 700 WP Insecticide, Mimic 240 SC Insecticide"
 |
