Chemistry:Edotreotide
| |
| Names | |
|---|---|
| IUPAC name
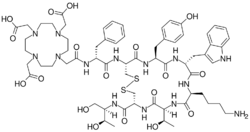
2-[4-[2-[[(2R)-1-[[(4R,7S,10S,13R,16S,19R)-10-(4-aminobutyl)-4-[[(2R,3R)-1,3-dihydroxybutan-2-yl]carbamoyl]-7-[(1R)-1-hydroxyethyl]-16-[(4-hydroxyphenyl)methyl]-13-(1H-indol-3-ylmethyl)-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentazacycloicos-19-yl]amino]-1-oxo-3-phenylpropan-2-yl]amino]-2-oxoethyl]-7,10-bis(carboxymethyl)-1,4,7,10-tetrazacyclododec-1-yl]acetic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C65H92N14O18S2 | |
| Molar mass | 1421.65 g·mol−1 |
| Pharmacology | |
| License data | |
| Pharmacology | |
| Legal status | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
| Clinical data | |
|---|---|
| Trade names | SomaKit TOC |
| Identifiers | |
| DrugBank | |
Edotreotide (USAN, also known as (DOTA0-Phe1-Tyr3) octreotide, DOTA-TOC, DOTATOC) is a substance which, when bound to various radionuclides, is used in the treatment and diagnosis of certain types of cancer.[3] When used therapeutically it is an example of peptide receptor radionuclide therapy.
Yttrium-90
A phase I clinical trial of yttrium-90 labelled edotreotide concluded in 2011,[4] aiming to investigated effects in young cancer patients (up to 25 years of age). Specific cancers being included in the trial include neuroblastoma, childhood brain tumours and gastrointestinal cancer.[5]
A phase II trial for the use of 90Y DOTA-TOC for patients with metastatic carcinoid, where octreotide treatment was no longer effective, also reported results in 2010.[6]

Yttrium-90 labeled edotreotide
Lutetium-177
Lutetium-177 labelled edotreotide (177Lu-DOTA-TOC), with the trade name Solucin, is the subject of a phase 3 clinical trial for treatment of GEP-NETs.[7][8] It was granted orphan drug designation by the European Medicines Agency in 2014.[9]
See also
- DOTA-TATE, a similar compound
References
- ↑ "SomaKit TOC 40 micrograms kit for radiopharmaceutical preparation - Summary of Product Characteristics (SmPC)". 2 July 2021. https://www.medicines.org.uk/emc/product/12722/smpc.
- ↑ "SomaKit TOC EPAR". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/somakit-toc.
- ↑ Martindale, The Extra Pharmacopoeia, 30th ed, p1161.
- ↑ S, O'Dorisio (17 June 2016) (in en). Radiolabeled Octreotide in Treating Children With Advanced or Refractory Solid Tumors. US National Library of Medicine. https://www.clinicaltrials.gov/ct2/show/NCT00049023. Retrieved 7 November 2020.
- ↑ "Phase I trial of 90Y-DOTATOC therapy in children and young adults with refractory solid tumors that express somatostatin receptors". Journal of Nuclear Medicine 51 (10): 1524–31. October 2010. doi:10.2967/jnumed.110.075226. PMID 20847174.
- ↑ "90Y-edotreotide for metastatic carcinoid refractory to octreotide". Journal of Clinical Oncology 28 (10): 1652–9. April 2010. doi:10.1200/JCO.2009.22.8585. PMID 20194865.
- ↑ "The therapeutic n.c.a. 177Lu-Edotreotide (Solucin)" (in en). ITM Isotopen Technologien München AG. https://itm-radiopharma.com/index.php?id=96.
- ↑ "A prospective, randomised, Controlled, Open-label, Multicentre phase III study to evaluate efficacy and safety of Peptide Receptor Radionuclide Therapy (PRRT) with Lutetium 177-Edotreotide compared to targeted molecular therapy with Everolimus in patients with inoperable, progressive, somatostatin receptor-positive (SSTR+), neuroendocrine tumours of gastroenteric or pancreatic origin (GEP-NET).". https://www.clinicaltrialsregister.eu/ctr-search/trial/2016-001897-13/GB.
- ↑ "EU/03/14/1269". 17 September 2018. https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu03141269.
External links
- "Edotreotide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/edotreotide.
 |
