Chemistry:Esculeoside A
| |
| Names | |
|---|---|
| IUPAC name
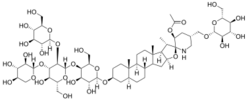
(2aS,2'S,3'S,4S,5'S,6aS,6bS,8aS,8bR,9S,11aS,12bR)-4-(((2R,3R,4R,5R,6R)-3,4-dihydroxy-5-(((2S,3R,4S,5R,6R)-5-hydroxy-6-(hydroxymethyl)-3-(((2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-4-(((2S,3R,4S,5R)-3,4,5-trihydroxytetrahydro-2H-pyran-2-yl)oxy)tetrahydro-2H-pyran-2-yl)oxy)-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)-6a,8a,9-trimethyl-5'-((((2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl)oxy)methyl)octadecahydrospiro[naphtho[2',1':4,5]indeno[2,1-b]furan-10,2'-piperidin]-3'-yl acetate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C58H95NO29 | |
| Molar mass | 1270.376 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Esculeoside A is a spirosolane-type glycoside with the molecular formula C58H95NO29.[1] The structure of this product is 3-Ο-β-lycotetraosyl (22S,23S,25S)-23-acetoxy-3β,27-dihydroxy-5α-spirosolane 27-Ο-β-D-glucopyranoside.[1] Fujiwara and colleagues were the first to isolate esculeoside A from the ripe fruit of the Cherry tomato in 2002. Esculeoside A, along with many other steroidal alkaloid glycosides, have been shown to possess cytotoxic activity that could result in a variety of potential health benefits for humans.
Synthesis
This natural product can be obtained using column chromatographies of high-porous polystyrene gels and reversed silica gels from a methanolic extract of many varieties of tomatoes.[2] It will appear as colorless needles when synthesized using this method.
Evidence suggests that α-tomatine is a precursor of esculeoside A.[3] In order for alpha tomatine to be converted to esculeoside A, isomerization of the F-ring is required. The mechanism for this reaction is unclear at this time but research from Iijima and colleagues in 2009 suggest a glycosylation step in the putative pathway from α-tomatine to esculeoside A depends on the plant hormone ethylene.[3][4]
Occurrence
Potatoes, eggplant, and tomatoes are all solanaceous plants that contain unique glycoalkaloids.[4] In the case of tomatoes, one of those unique glycoalkaloids is esculeoside A. A tomato saponin, esculeoside A, is found in quantities four times that of lycopene in ripe tomatoes.[5]
Potential health benefits
Studies have shown esculeoside A may be metabolized into derivatives that perform various beneficial activities in the human body including anti-osteoporosis, anti-menopausal disorder and anti-tumor activities.[5] Recent studies in mice have shown a potential link between esculeoside A and cholesterol levels. In one study, esculeoside A administered to mice reduced serum levels of LDL cholesterol and triglycerides by 25-45% without impacting the rates of HDL cholesterol.[1] The potential health benefits of esculeoside A appear to change with factors such as the age of the tomato fruit, the heat used in processing tomatoes, and the pH used in processing.[6] The highest amounts of esculeoside A were found in the outer skin and wall (pericarp wall) of the tomato fruit. Mature tomatoes tended to show higher amounts of esculeoside A than extracts taken from immature tomatoes. Extracts of esculeoside A in the Katsumata study were shown to be stable when heated until the point of 225 °C. This same study found esculeoside A extracts in water at pH 7-11 were stable throughout the heat sterilization process but unstable under acidic conditions.[6] Research has also shown esculeoside A amounts increase when tomatoes are treated with the phytohormone, ethylene.[4] Collectively, research suggests daily intake of esculeoside A from tomatoes could have many benefits.
References
- ↑ 1.0 1.1 1.2 Nohara, Toshihiro; Ono, M.; Ikeda, T.; Fujiwara, Y.; El-Aasr, M. (2010). "The Tomato Saponin, Esculeoside A". Journal of Natural Products 73 (10): 1734–1741. doi:10.1021/np100311t. PMID 20853874.
- ↑ Fujiwara, Y.; Takaki, A.; Uehara, Y.; Ikeda, T.; Okawa, M.; Yamauchi, K.; Ono, M.; Yoshimitsu, H. et al. (2004). "Tomato steroidal alkaloid glycosides, esculeosides A and B, from ripe fruits". Tetrahedron 60 (22): 4915–4920. doi:10.1016/j.tet.2004.03.088.
- ↑ 3.0 3.1 Yamanaka, T.; Vincken, J.; Zuilhof, H.; Legger, A.; Takada, N.; Gruppen, H. (2009). "C22 Isomerization in a-Tomatine-to-Esculeoside A Conversion during Tomato Ripening Is Driven by C27 Hydroxylation of Triterpenoidal Skeleton". Journal of Agricultural and Food Chemistry 57 (9): 3786–3791. doi:10.1021/jf900017n. PMID 19415927.
- ↑ 4.0 4.1 4.2 Iijima, Y.; Fujiwara, Y.; Tokita, T.; Ikeda, T.; Nohara, T.; Aoki, K.; Shibata, D. (2009). "Involvement of Ethylene in the Accumulation of Esculeoside A during Fruit Ripening of Tomato (Solanum lycopersicum)". Journal of Agricultural and Food Chemistry 57 (8): 3247–3252. doi:10.1021/jf8037902. PMID 19284799.
- ↑ 5.0 5.1 Manabe, H.; Murakami, Y.; El-Aasr, M.; Ikeda, T.; Fujiwara, Y.; Ono, M.; Nohara, T. (2010). "Content variations of the tomato saponin Esculeoside A in various processed tomatoes". Journal of Natural Medicine 65 (1): 176–179. doi:10.1007/s11418-010-0443-4. PMID 20652644.
- ↑ 6.0 6.1 Katsumata, A.; Kimura, M.; Saigo, H.; Aburaya, K.; Nakano, M.; Ikeda, T.; Fujiwara, Y.; Nagai, R. (2011). "Changes in Esculeoside A Content in Different Regions of the Tomato Fruit during Maturation and Heat Processing". Journal of Agricultural and Food Chemistry 59 (8): 4104–4110. doi:10.1021/jf104025p. PMID 21395308.
External links
 |


