Chemistry:Tomatine
| |
| Names | |
|---|---|
| IUPAC name
(22S,25S)-5α-spirosolan-3β-yl β-D-glucopyranosyl-(1→2)-[β-D-xylopyranosyl-(1→3)]-β-D-glucopyranosyl-(1→4)-β-D-galactopyranoside [1]
| |
| Other names
Tomatine, Tomatin, Lycopersicin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C50H83NO21 [2] | |
| Molar mass | 1034.18816 [3] |
| Appearance | crystalline solid |
| Melting point | 263-268 °C [4] |
| insoluble but soluble in methanol, ethanol, dioxane and propylene glycol[4] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
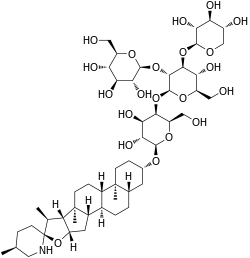
Tomatine (sometimes called tomatin or lycopersicin) is a glycoalkaloid, found in the stems and leaves of tomato plants, and in the fruits at much lower concentrations. Chemically pure tomatine is a white crystalline solid at standard temperature and pressure.[1][5]
Tomatine is sometimes confused with the glycoalkaloid solanine.[6]
History
Tomatoes were brought to Europe in the early 1500s. The English botanist John Gerard was one of the first cultivators of the tomato plant. In his publication Grete Herball, he considered tomatoes poisonous due to their levels of what would later be called tomatine, plus high acid content. Consequently, tomatoes were generally not eaten in Britain until the mid-18th century.[7][better source needed]
In 1837, the first medicinal tomato pills were advertised in the United States because of their positive effects upon the biliary organs. The product “Phelp’s Compound Tomato Pills” was extracted from the tomato plant, and contained tomatine. The pills were made by the medic Guy R. Phelps, who stated that the alkaloid tomatine was one of the most useful discoveries ever made. Tomatine then was said to be an antidote to mercury.[8]
In the mid 20th century, scientists from the US Department of Agriculture were the first to isolate tomatine from the wild tomato species Lycopersicon pimpinellifolium and the cultured species Lycopersicon esculentum.[9][10]
Structure and biosynthesis

Alpha-tomatine (α-tomatine) belongs to the compound group steroidal glycoalkaloids. These compounds consist of an aglycon, which is a cholesterol derivative, and a carbohydrate chain, which in the case of α-tomatine consists of two d-glucose units, a d-galactose unit, and a d-xylose unit.[12] In α-tomatine, the tetrasaccharide called lycotetraose is attached to the O-3 of the steroidal aglycone.[13] At first it was thought that the synthesis of steroidal alkaloids only involved multiple steps of hydroxylation, oxidation and amination of cholesterol with arginine as the source of the incorporated nitrogen. Later the glycoalkaloid metabolism genes were discovered.[12] These genes produce the glycoalkaloid metabolism enzymes, which are responsible for the synthesis of steroidal alkaloid aglycones in potato and tomato plants.[12] The reaction these enzymes perform are shown in the figure 1.
Mechanism of action
Tomatine may play a major role in resistance of the tomato plant against fungal, microbial, insect, and herbivoral attack.[citation needed]
The effects of the glycoalkaloids (to which tomatine belongs), can be divided in two main parts: the disruption of cellular membranes and the inhibition of the enzyme acetylcholinesterase. Tomatine is responsible in tomato plants for resistance against for example the Colorado beetle and to snails.[14] It is also a defense against fungi.[15][16]
Membrane disruption
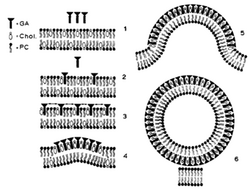
The membrane disruptive properties of tomatine are caused by the ability to form 1:1 complexes with cholesterol. A possible mechanism of the membrane disruption by glycoalkaloids is displayed in figure 2. First, the aglycon part of tomatine binds reversibly to sterols in the membrane (figure 2, part 2). When this reaches a certain density, the glycosidic residues of the glycoalkaloids interact with each other by electrostatic interactions. This interaction catalyzes the development of an irreversible matrix of glycoalkaloid-sterol complexes (figure 2, part 4). In this way, the sterols from the external membrane are immobilized and membrane budding will arise. Tubular structures are formed, because of the structure of tomatine (figure 2, part 6).[14][17] This membrane disruption causes cell death by cell leakage.[14] Also, the disrupted membrane has an influence on sodium transport, by altering the membrane potential and reducing active sodium transport. When tomatine is orally ingested, the brush border of the intestine is damaged by the membrane-disruptive properties of tomatine, so increased uptake of macromolecules occurs. This damage to the epithelial barriers is dose-dependent.[14][17]
Tomatine is considered to be a fungitoxic compound, as it completely inhibits mycelial growth of the fungi C. orbiculare (MC100=2.0 mM), S. linicola (MC100=0.4 mM), and H. turcicum (MC100=0.13 mM). For the inhibition at a low pH, much more tomatine is required, so the compound is more effectively fungitoxic at a high pH, when the alkaloid is unprotonated. The unprotonated form of tomatine forms complexes with sterols such as cholesterol, which may cause disruption of cell membrane and changes in membrane permeability.[18]
Tomatine is effective against fungi at pH 8 but not at pH 4. A possible explanation for this is that the tomatine only in the deprotonated form binds to cholesterol to form the earlier mentioned complexes.[15] Tomatine disrupts liposome membranes containing 3-β-hydroxy sterol, while liposomes without 3-β-hydroxy sterols are resistant to membrane disruption.[16] Tomatine inhibits also the fungal types Ph. infestans and Py. aphanidermatum, which do not have any sterols in their membranes, so another mechanism of action must be present.[15]
Inhibition of acetylcholinesterase
The other known action of the compound is the pH-dependent competitive inhibition of the enzyme acetylcholinesterase.[14][15] The majority of synthetic pesticides used in agriculture work by inhibition of acetylcholinesterase is to kill insects.[19]
Metabolism
Even now, little is known about the bioavailability, pharmacokinetics and metabolism of the glycoalkaloids in humans.[14] One important factor is the poor uptake of tomatine into general blood circulation. When tomatine is orally ingested, much tomatine may form complexes with cholesterol from the other food present in the stomach. The complexes of tomatine and cholesterol are not absorbed in the intestine, but are excreted.[15] For the complexation with cholesterol to occur, the presence of a carbohydrate chain is essential. The aglycon tomatidine, which is tomatine without the sugars, does not form the complexes.[14][17] The complexation probably occurs in the duodenum, because the acidic conditions in the stomach itself lead to protonation of the tomatine, and the protonated form of tomatine does not bind to cholesterol.[15]
Hydrolysis of tomatine likely takes place, but whether it is acid- or glycosidase-catalyzed is not known.[15] The hydroxylation of tomatine likely leads to the formation of tomatidine, which is the aglycon of tomatine. Tomatidine is a metabolite which may not be completely nontoxic; it could have effects on the human body.[15]
Fungal tomatinase enzymes can transform tomatine to deactivate it. Detoxification can take place by removing one glucose residue. Other fungal species hydrolyze tomatine to the less toxic aglycon tomatidine by removing all the sugar residues. Tomatidine can still inhibit some fungal species, but is less toxic than tomatine. Fungi use diverse pathways for the hydrolysis of tomatine. Also, the level of toxicity depends on the type of fungus.[16][20] The metabolite tomatidine can be hydrolyzed further by membrane-bound CYP-450 oxygenases.[15]
Uses
Tomatine has been used as a reagent in analytical chemistry for precipitating cholesterol from solution.[21] Also, tomatine is known to be an immune adjuvant in connection with certain protein antigens.[22]
Toxicity
The possible risks of tomatine for humans have not been formally studied, so no NOAEL can be deduced. The toxicity of tomatine has only been studied on laboratory animals. The symptoms of acute tomatine poisoning in animals are similar to the symptoms of poisoning by solanine, a potato glycoalkaloid. These symptoms include vomiting, diarrhea, abdominal pain, drowsiness, confusion, weakness, and depression.[23] Generally, tomatine is regarded to cause less toxic effects to mammals than other alkaloids such as solanine.[24]
The human consumption of moderate amounts of tomatine seems to occur without notable toxic effects. This is reinforced by the widespread consumption of “pickled green” and “fried green tomatoes” and the consumption of high-tomatine tomatoes (a variant of L. esculentum var. cerasiforme, better known as the "cherry tomato", indigenous to Peru) with very high tomatine content (in the range of 500–5000 μg/kg of dry weight).[25]
New York Times food science writer Harold McGee found scant evidence for tomato toxicity in the medical and veterinary literature, and observed that dried tomato leaves (which contain higher concentrations of alkaloids than the fruits) are occasionally used as a food flavoring or garnish, without problems. He also reported that an adult human would probably have to eat over half a kilogram of tomato leaves to ingest a toxic (not necessarily lethal) dose.[6]
See also
References
- ↑ 1.0 1.1 EBI Web Team. "tomatine (CHEBI:9630)". http://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:9630.
- ↑ 1.http://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+hsdb:@term+@rn+@rel+17406-45-0
- ↑ US Department of Health and Human Services, Public Health Service, Center for Disease Control, National Institute for Occupational Safety Health. Registry of Toxic Effects of Chemical Substances (RTECS). National Library of Medicine's current MEDLARS file., p. 83/8212
- ↑ 4.0 4.1 The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 1228
- ↑ Degtyarenko, K.; De Matos, P.; Ennis, M.; Hastings, J.; Zbinden, M.; McNaught, A.; Alcantara, R.; Darsow, M. et al. (2007). "ChEBI: A database and ontology for chemical entities of biological interest". Nucleic Acids Research 36 (Database issue): D344–50. doi:10.1093/nar/gkm791. PMID 17932057.
- ↑ 6.0 6.1 McGee, Harold (July 29, 2009). "Accused, Yes, but Probably Not a Killer". The New York Times. https://www.nytimes.com/2009/07/29/dining/29curi.html.
- ↑ "Tomatoes Culinary History – Resource – Smart Kitchen – Online Cooking School". http://smartkitchen.com/resources/tomato-s-culinary-history.
- ↑ Andrew F. Smith; The tomato in America: Early History, Culture, and Cookery; University of South Carolina Press, 1994; 112.
- ↑ Fontaine, T. D.; Irving, G. W., Jr.; Ma, R.; Poole, J. B.; Doolittle, S. P; Isolation and partial characterization of crystalline tomatine, an antibiotic agent from the tomato plant; Arch. Biochem. 1948; 18, 467-475.
- ↑ Fontaine, T. D., Ard, J. S., Ma, R. M.; Tomatidine, a steroid secondary amine; J. Am. Chem. SOC, 1951; 73, 878-879.
- ↑ 11.0 11.1 Cárdenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. (2015). "The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism". Phytochemistry 113: 24–32. doi:10.1016/j.phytochem.2014.12.010. PMID 25556315. Bibcode: 2015PChem.113...24C.
- ↑ 12.0 12.1 12.2 Cárdenas, P.D.; Sonawane, P.D.; Heinig, U.; Bocobza, S.E.; Burdman, S.; Aharoni, A. (2015). "The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism". Phytochemistry 113: 24–32. doi:10.1016/j.phytochem.2014.12.010. PMID 25556315. Bibcode: 2015PChem.113...24C.
- ↑ Jones, Nigel A.; Nepogodiev, Sergey A.; Field, Robert A. (2005). "Efficient synthesis of methyl lycotetraoside, the tetrasaccharide constituent of the tomato defence glycoalkaloid α-tomatine". Organic & Biomolecular Chemistry 3 (17): 3201–6. doi:10.1039/B508752J. PMID 16106302.
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Milner, Sinead Eileen, et al. "Bioactivities of glycoalkaloids and their aglycones from Solanum species." Journal of agricultural and food chemistry 59.8 (2011): 3454-3484.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 15.6 15.7 15.8 Friedman, Mendel; Tomato glycoalkaloids: role in the plant and in the diet; Journal of Agricultural and Food Chemistry 50.21, 2002; 5751-5780.
- ↑ 16.0 16.1 16.2 Hoagland, Robert E.; Toxicity of tomatine and tomatidine on weeds, crops and phytopathogenetic fungi.; Allelopathy J 23.2, 2009; 425-436.
- ↑ 17.0 17.1 17.2 Keukens, Erik AJ, et al; Dual specificity of sterol-mediated glycoalkaloid induced membrane disruption; Biochimica et Biophysica Acta (BBA) - Biomembranes 1110.2, 1992; 127-136.
- ↑ Arneson, P.A., Durbin, R.D.; Studies on the Mode of Action of Tomatine as a Fungitoxic Agent.; USDA Pioneering Research Laboratory, 1967.
- ↑ Bushway, Rodney J., Sharon A. Savage, and Bruce S. Ferguson; Inhibition of acetyl cholinesterase by solanaceous glycoalkaloids and alkaloids; American Potato Journal 64.8, 1987;
- ↑ Arneson, P. A., and R. D. Durbin.; Studies on the mode of action of tomatine as a fungitoxic agent.; Plant physiology 43.5, 1968; 683-686.
- ↑ Cayen, M. N. (1971). "Effect of dietary tomatine on cholesterol metabolism in the rat". Journal of Lipid Research 12 (4): 482–90. doi:10.1016/S0022-2275(20)39498-0. PMID 4362143. http://www.jlr.org/cgi/pmidlookup?view=long&pmid=4362143.
- ↑ Heal, K. G.; Taylor-Robinson, A. W. (2010). "Tomatine Adjuvantation of Protective Immunity to a Major Pre-erythrocytic Vaccine Candidate of Malaria is Mediated via CD8+ T Cell Release of IFN-γ". Journal of Biomedicine and Biotechnology 2010: 834326. doi:10.1155/2010/834326. PMID 20300588.
- ↑ Morris, S.C., Lee, T.H; The toxicity and teratogenicity of Solanaceae glycoalkaloids, particularly those of the potato (Solanum tuberosum): a review.; Food Techn. Aust., 1984; 118-124.
- ↑ Rick, C. M.; Uhlig, J. W.; Jones, A. D. (1994). "High alpha-tomatine content in ripe fruit of Andean Lycopersicon esculentum var. Cerasiforme: Developmental and genetic aspects". Proceedings of the National Academy of Sciences of the United States of America 91 (26): 12877–12881. doi:10.1073/pnas.91.26.12877. PMID 7809139. Bibcode: 1994PNAS...9112877R.
- ↑ Rick, C. M., Uhlig, J. W., Jones, A. D.; High R-tomatine content in ripe fruit of Andean Lycopersicon esculentum Var. cerasiforme: developmental and genetic aspects.; Proc. Natl. Acad. Sci., 1994; 91, 12877-12881.
External links
