Chemistry:Ethyl maltol
From HandWiki
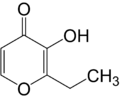
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Ethyl-3-hydroxy-4H-pyran-4-one | |
| Other names
2-Ethyl-3-hydroxy-4-pyranone
2-Ethyl pyromeconic acid 2-Ethyl-3-hydroxy-4-pyrone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C7H8O3 | |
| Molar mass | 140.138 g·mol−1 |
| Appearance | White crystalline powder |
| Melting point | 85 to 95 °C (185 to 203 °F; 358 to 368 K)[1] |
| Boiling point | 161 °C (322 °F; 434 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Ethyl maltol is an organic compound that is a common flavourant in some confectioneries. It is related to the more common flavorant maltol by replacement of the methyl group by an ethyl group.[2] It is a white solid with a sweet smell that can be described as caramelized sugar or as caramelized fruit.
The conjugate base derived from ethylmaltol, again like maltol, has a high affinity for iron, forming a red coordination complex[citation needed]. In such compounds, the heterocycle is a bidentate ligand.
Original patent:[3]
References
- ↑ Ethyl maltol at Sigma-Aldrich
- ↑ Erich Lück and Gert-Wolfhard von Rymon Lipinski "Foods, 3. Food Additives" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a11_561
- ↑ Charles R Stephens Jr & Robert P Allingham, U.S. Patent 3,446,629 (1969 to Pfizer Inc).
 |

