Chemistry:Ethyl methacrylate
From HandWiki
Short description: Organic compound
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethyl 2-methylprop-2-enoate | |
| Other names
Ethyl 2-methylpropenoate, Acryester E, Acryester BMA
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 2277 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H10O2 | |
| Molar mass | 114.144 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.9135 g/cm3 |
| Boiling point | 117 °C (243 °F; 390 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H225, H315, H317, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264, P271, P272, P280, P302+352, P303+361+353, P304+340, P305+351+338, P312, P321, P332+313, P333+313, P337+313, P362, P363, P370+378, P403+233, P403+235 | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
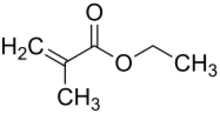
Ethyl methacrylate is the organic compound with the formula C2H5O2CC(CH3)=CH2. A colorless liquid, it is a common monomer for the preparation of acrylate polymers.[1] It is typically polymerized under free-radical conditions.[2]
Ethyl methacrylate was first obtained by treating 2-hydroxyisobutyric acid with phosphorus pentachloride in an apparent dehydration reaction.[3]
Environmental issues and health hazards
The acute toxicity of the related butyl methacrylate is the LD50 is 20 g/kg (oral, rat). Acrylate esters irritate the eyes and can cause blindness.[1]
See also
References
- ↑ 1.0 1.1 Bauer, Jr., William (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a16_441..
- ↑ Granel, C.; Dubois, Ph.; Jérôme, R.; Teyssié, Ph. (1996). "Controlled Radical Polymerization of Methacrylic Monomers in the Presence of a Bis(ortho-chelated) Arylnickel(II) Complex and Different Activated Alkyl Halides". Macromolecules 29 (27): 8576–8582. doi:10.1021/ma9608380. Bibcode: 1996MaMol..29.8576G.
- ↑ E. Frankland, B. F. Duppa (1865). "Untersuchungen über Säuren aus der Acrylsäure-Reihe; 1) Umwandlung der Säuren aus der Milchsäure-Reihe in die der Acrylsäure-Reihe". Justus Liebigs Annalen der Chemie 136: 12. doi:10.1002/jlac.18651360102. https://zenodo.org/record/1427247.
 |


