Chemistry:Euxanthone
From HandWiki
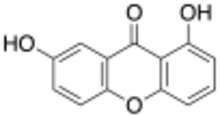
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,7-Dihydroxy-9H-xanthen-9-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | |
| 207044 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C13H8O4 | |
| Molar mass | 228.203 g·mol−1 |
| Melting point | 240 °C (464 °F; 513 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Euxanthone is a naturally occurring xanthonoid, an organic compound with the molecular formula C13H8O4. It can be synthesized from gentisic acid, β-resorcylic acid, and acetic anhydride.[1] It occurs naturally in many plant species. Commercial production is from purified root extract of Polygala tenuifolia.[2] It has been investigated for bioactive properties.[3][4]
References
- ↑ Baer, N.S.. "Indian Yellow". Artists' pigments : a handbook of their history and characteristics. Washington: National Gallery of Art. pp. 25 Fig. 4D Synthesis of euxanthone. https://www.nga.gov/research/publications/pdf-library/artists-pigments-vol-1.html.
- ↑ "Polygala tenuifolia Willd. -- ChemFaces". http://www.chemfaces.com/direct/Polygala-tenuifolia-Willd-20090.html.
- ↑ Naidu, M.; Kuan, C.-Y.K.; Lo, W.-L.; Raza, M.; Tolkovsky, A.; Mak, N.-K.; Wong, R.N.-S.; Keynes, R. (2007). "Analysis of the action of euxanthone, a plant-derived compound that stimulates neurite outgrowth". Neuroscience 148 (4): 915–924. doi:10.1016/j.neuroscience.2007.07.037. ISSN 0306-4522. PMID 17825492.
- ↑ Câmara, D.V.; Lemos, V.S.; Santos, M.H.; Nagem, T.J.; Cortes, S.F. (2010). "Mechanism of the vasodilator effect of Euxanthone in rat small mesenteric arteries". Phytomedicine 17 (8–9): 690–692. doi:10.1016/j.phymed.2009.12.003. ISSN 0944-7113. PMID 20097048.
 |

