Chemistry:Glycopeptide
Glycopeptides are peptides that contain carbohydrate moieties (glycans) covalently attached to the side chains of the amino acid residues that constitute the peptide.
Over the past few decades it has been recognised that glycans on cell surface (attached to membrane proteins or lipids) and those bound to proteins (glycoproteins) play a critical role in biology. For example, these constructs have been shown to play important roles in fertilization,[1] the immune system,[2] brain development,[3] the endocrine system,[3] and inflammation.[3][4][5]
The synthesis of glycopeptides provides biological probes for researchers to elucidate glycan function in nature and products that have useful therapeutic and biotechnological applications.[clarification needed][citation needed]
Glycopeptide linkage variety
N-Linked glycans
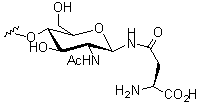
N-Linked glycans derive their name from the fact that the glycan is attached to an asparagine (Asn, N) residue, and are amongst the most common linkages found in nature. Although the majority of N-linked glycans take the form GlcNAc-β-Asn[6] other less common structural linkages such as GlcNac-α-Asn[7] and Glc-Asn[8] have been observed. In addition to their function in protein folding and cellular attachment, the N-liked glycans of a protein can modulate the protein's function, in some cases acting as an on-off switch.[5]
O-Linked glycans
O-Linked glycans are formed by a linkage between an amino acid hydroxyl side chain (usually from serine or threonine) with the glycan. The majority of O-linked glycans take the form GlcNac-β-Ser/Thr or GalNac-α-Ser/Thr.[6]
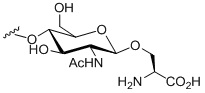
C-Linked glycans
Of the three linkages the least common and least understood are C-linked glycans. The C-linkage refers to the covalent attachment of mannose to a tryptophan residue. An example of a C-linked glycan is α-mannosyl tryptophan.[9][10]
Glycopeptide synthesis
Several methods have been reported in the literature for the synthesis of glycopeptides. Of these methods the most common strategies are listed below.
Solid phase peptide synthesis
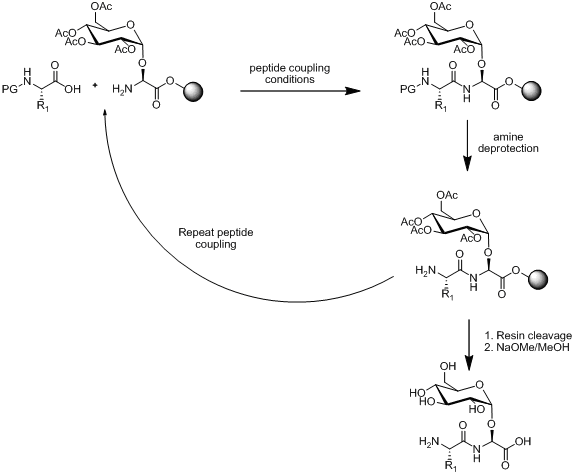
Within solid phase peptide synthesis (SPPS) there exist two strategies for the synthesis of glycopeptides, linear and convergent assembly. Linear assembly relies on the synthesis of building blocks and then the use of SPPS to attach the building block together. An outline of this approach is illustrated below.
Several methods exist for the synthesis of monosaccharide amino acid building block as illustrated below.
Provided the monosaccharide amino acid building block is stable to peptide coupling conditions, amine deprotection conditions and resin cleavage. Linear assembly remains a popular strategy for the synthesis of glycopeptides with many examples in the literature.[13][14][15]
In the convergent assembly strategy a peptide chain and glycan residue are first synthesis separately. Then the glycan is glycosylated onto a specific residue of the peptide chain. This approach is not as popular as the linear strategy due to the poor reaction yields in the glycosylation step.[16]
Another strategy to produce glycopeptide libraries is using Glyco-SPOT synthesis technique.[17] The technique extends the existing method of SPOT synthesis.[18] In this method, libraries of glycopeptides are produced on a cellulose surface (e.g. filter paper) which acts as the solid phase. The glycopeptides are produced by spotting FMOC protected amino acids allowing the synthesis to be performed at microgram (nanomole) scale using very small amounts of glycoamino acids. The scale of this technique can be an advantage for creating libraries for screening by using less amounts of glycoamino acids per peptide. However to produce larger quantities of glycopeptides traditional resin-based solid phase techniques would be better.
Native chemical ligation
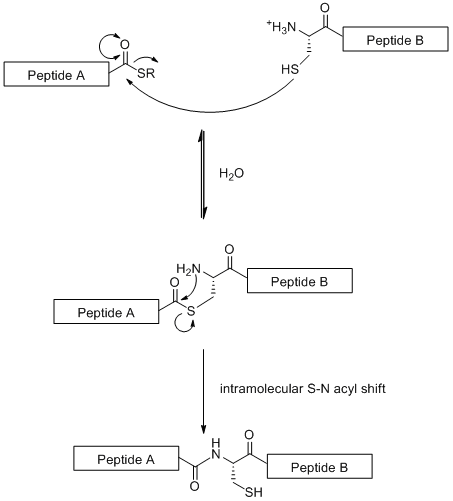
Native chemical ligation (NCL) is a convergent synthetic strategy based on the linear coupling of glycopeptide fragments. This technique makes use of the chemoselective reaction between a N-terminal cysteine residue on one peptide fragment with a thio-ester on the C-terminus of the other peptide fragment[19] as illustrated below.
Unlike standard SPPS (which is limited to 50 amino acid residue) NCL allows the construction of large glycopeptides. However the strategy is limited by the fact that it requires a cysteine residue at N-terminus, an amino acid residue that is rare in nature.[19] However this problem has partly been address by the selective desulfurization of the cysteine residue to an alanine.[20]
See also
References
- ↑ Talbot P.; Shur B. D.; Myles D. G. (2003). "Cell adhesion and fertilization: Steps in oocyte transport, sperm-zona pellucida interactions, and sperm-egg fusion". Biology of Reproduction 68 (1): 1–9. doi:10.1095/biolreprod.102.007856. PMID 12493688.
- ↑ Rudd P. M.; Elliott T.; Cresswell P.; Wilson I. A.; Dwek R. A. (2001). "Glycosylation and the immune system". Science 291 (5512): 2370–2376. doi:10.1126/science.291.5512.2370. PMID 11269318. Bibcode: 2001Sci...291.2370R.
- ↑ 3.0 3.1 3.2 Varki A (1993). "Biological Roles of Oligosaccharides - All of the Theories Are Correct". Glycobiology 3 (2): 97–130. doi:10.1093/glycob/3.2.97. PMID 8490246.
- ↑ Bertozzi C. R.; Kiessling L. L. (2001). "Chemical glycobiology". Science 291 (5512): 2357–2364. doi:10.1126/science.1059820. PMID 11269316. Bibcode: 2001Sci...291.2357B.
- ↑ 5.0 5.1 "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity". J Autoimmun 57 (6): 1–13. 2015. doi:10.1016/j.jaut.2014.12.002. PMID 25578468.
- ↑ 6.0 6.1 Vliegenthart J. F. G.; Casset F. (1998). "Novel forms of protein glycosylation". Current Opinion in Structural Biology 8 (5): 565–571. doi:10.1016/s0959-440x(98)80145-0. PMID 9818259.
- ↑ Shibata S.; Takeda T.; Natori Y. (1988). "The Structure of Nephritogenoside - a Nephritogenic Glycopeptide with Alpha-N-Glycosidic Linkage". Journal of Biological Chemistry 263 (25): 12483–12485. doi:10.1016/S0021-9258(18)37780-9. PMID 3410849. http://www.jbc.org/content/263/25/12483.abstract.
- ↑ Wieland F.; Heitzer R.; Schaefer W. (1983). "Asparaginylglucose - Novel Type of Carbohydrate Linkage". Proceedings of the National Academy of Sciences of the United States of America 80 (18): 5470–5474. doi:10.1073/pnas.80.18.5470. PMID 16593364. Bibcode: 1983PNAS...80.5470W.
- ↑ Debeer T.; Vliegenthart J. F. G.; Loffler A.; Hofsteenge J. (1995). "The Hexopyranosyl Residue That Is C-Glycosidically Linked to the Side-Chain of Tryptophan-7 in Human Rnase U-S Is Alpha-Marmopyranose". Biochemistry 34 (37): 11785–11789. doi:10.1021/bi00037a016. PMID 7547911.
- ↑ Ihara, Yoshito; Inai, Yoko; Ikezaki, Midori; Matsui, In-Sook L.; Manabe, Shino; Ito, Yukishige (2014). "C-Mannosylation: A Modification on Tryptophan in Cellular Proteins". Glycoscience: Biology and Medicine: 1–8. doi:10.1007/978-4-431-54836-2_67-1. ISBN 978-4-431-54836-2.
- ↑ Jansson A. M.; Meldal M.; Bock K. (1990). "The Active Ester N-Fmoc-3-O-[Ac4-Alpha-D-Manp-(1-]2)-Ac3-Alpha-D-Manp-1-]-Threonine-O-Pfp as a Building Block in Solid-Phase Synthesis of an O-Linked Dimannosyl Glycopeptide". Tetrahedron Letters 31 (48): 6991–6994. doi:10.1016/s0040-4039(00)97224-1.
- ↑ Elofsson M.; Walse B.; Kihlberg J. (1991). "Building-Blocks for Glycopeptide Synthesis – Glycosylation of 3-Mercaptopropionic Acid and Fmoc Amino-Acids with Unprotected Carboxyl Groups". Tetrahedron Letters 32 (51): 7613–7616. doi:10.1016/0040-4039(91)80548-k.
- ↑ Li H. G.; Li B.; Song H. J.; Breydo L.; Baskakov I. V.; Wang L. X. (2005). "Chemoenzymatic synthesis of HIV-1V3 glycopeptides carrying two N-glycans and effects of glycosylation on the peptide domain". Journal of Organic Chemistry 70 (24): 9990–9996. doi:10.1021/jo051729z. PMID 16292832.
- ↑ Yamamoto N.; Takayanagi Y.; Yoshino A.; Sakakibara T.; Kajihara Y. (2007). "An approach for a synthesis of asparagine-linked sialylglycopeptides having intact and homogeneous complex-type undecadisialyloligosaccharides". Chemistry: A European Journal 13 (2): 613–625. doi:10.1002/chem.200600179. PMID 16977655.
- ↑ Shao N.; Xue J.; Guo Z. W. (2003). "Chemical synthesis of CD52 glycopeptides containing the acid-labile fucosyl linkage". Journal of Organic Chemistry 68 (23): 9003–9011. doi:10.1021/jo034773s. PMID 14604374.
- ↑ Gamblin D. P.; Scanlan E. M.; Davis B. G. (2009). "Glycoprotein Synthesis: An Update". Chemical Reviews 109 (1): 131–163. doi:10.1021/cr078291i. PMID 19093879.
- ↑ Mehta, AY; Veeraiah, RKH; Dutta, S; Goth, CK; Hanes, MS; Gao, C; Stavenhagen, K; Kardish, R et al. (29 June 2020). "Parallel Glyco-SPOT Synthesis of Glycopeptide Libraries.". Cell Chemical Biology 27 (9): 1207–1219.e9. doi:10.1016/j.chembiol.2020.06.007. PMID 32610041.
- ↑ Hilpert, K; Winkler, DF; Hancock, RE (2007). "Peptide arrays on cellulose support: SPOT synthesis, a time and cost efficient method for synthesis of large numbers of peptides in a parallel and addressable fashion.". Nature Protocols 2 (6): 1333–49. doi:10.1038/nprot.2007.160. PMID 17545971.
- ↑ 19.0 19.1 Nilsson B. L.; Soellner M. B.; Raines R. T. (2005). "Chemical synthesis of proteins". Annual Review of Biophysics and Biomolecular Structure 34: 91–118. doi:10.1146/annurev.biophys.34.040204.144700. PMID 15869385.
- ↑ Wan Q.; Danishefsky S. J. (2007). "Free Radical Based, Specific Desulfurization of Cysteine: A Powerful Advance in the Synthesis of Polypeptides and Glycopolypeptides". Angew. Chem. 119 (48): 9408–9412. doi:10.1002/ange.200704195. PMID 18046687. Bibcode: 2007AngCh.119.9408W.
Further reading
- Emanual Maverakis. "Glycans in the immune system and The Altered Glycan Theory of Autoimmunity". http://ac.els-cdn.com/S0896841114001759/1-s2.0-S0896841114001759-main.pdf?_tid=fbc3820c-0881-11e5-b633-00000aacb362&acdnat=1433179187_223619b43246b42b6d0f8c696bf10ac7.
External links
 |