Chemistry:Harmol
From HandWiki
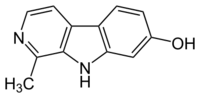
| |
| Names | |
|---|---|
| IUPAC name
1-Methyl-2,9-dihydropyrido[3,4-b]indol-7-one
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C12H10N2O | |
| Molar mass | 198.225 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Harmol is a chemical compound classified as a β-carboline.[1][2][3] It is readily formed in vivo in humans by O-demethylation of harmine.[4]
See also
References
- ↑ "Harmol induces autophagy and subsequent apoptosis in U251MG human glioma cells through the downregulation of survivin". Oncol Rep 29 (4): 1333–42. 2013. doi:10.3892/or.2013.2242. PMID 23338618.
- ↑ "Transcriptional and posttranslational inhibition of dioxin-mediated induction of CYP1A1 by harmine and harmol". Toxicol Lett 208 (1): 51–61. 2012. doi:10.1016/j.toxlet.2011.09.030. PMID 22001777.
- ↑ "The β-carboline alkaloid harmol induces cell death via autophagy but not apoptosis in human non-small cell lung cancer A549 cells.". Biol Pharm Bull 34 (8): 1264–72. 2011. doi:10.1248/bpb.34.1264. PMID 21804216.
- ↑ "The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro". Sci. Rep. 7 (1): 5309. 2017. doi:10.1038/s41598-017-05407-9. PMID 28706205. Bibcode: 2017NatSR...7.5309M.
 |
