Chemistry:Harmine
Harmine, also known as banisterine or telepathine among other synonyms, is a β-carboline and a harmala alkaloid which has hallucinogenic effects and monoamine oxidase inhibitor (MAOI) activity.[1] It occurs in a number of different plants, most notably Peganum harmala and Banisteriopsis caapi.[2] Harmine reversibly inhibits monoamine oxidase A (MAO-A), an enzyme which breaks down monoamines, making it a reversible inhibitor of monoamine oxidase A (RIMA). Harmine does not inhibit MAO-B.[3]
The biosynthesis of harmine likely begins with L-tryptophan, which is decarboxylated to tryptamine—an intermediate also used in serotonin synthesis—before undergoing a series of reactions to form harmine, with feeding experiments supporting tryptamine’s role as an intermediate rather than a primary precursor. It is essential for enabling the oral activity of DMT in ayahuasca and is also used as a fluorescent pH indicator and in PET imaging to study MAO-A-related brain disorders.
Pharmaceutical-grade harmine hydrochloride is safe and well-tolerated at oral doses below 2.7 mg/kg in healthy adults, with higher doses causing mild to moderate gastrointestinal and neurological side effects and limited psychoactive effects. It is found in various plants—including tobacco, Passiflora species, lemon balm, and several Banisteriopsis species—as well as in some butterflies of the Nymphalidae family. Harmine was first isolated and named by in 1848 from Peganum harmala seeds, later identified in Banisteriopsis caapi under various names, with its structure determined in 1927. Recent patents focus on creating harmine derivatives with reduced toxicity.
Use and effects
Hallucinogen
Harmine is a hallucinogen at reported doses of 25 to 75 mg subcutaneously, 150 to 200 mg intravenously, and 300 mg or more orally.[1][4] However, in other reports, hallucinogenic effects were minimal at doses of up to 960 mg orally and 750 mg sublingually.[1][4] The effects of harmine include euphoria, hallucinogenic effects, confusion, drowsiness, sleepiness, perceptual disturbances, closed-eye visuals, vertigo, lightheadedness, ataxia, speech impairment, and unpleasantness.[1] The hallucinogenic effects of harmine and other β-carbolines are said to be qualitatively distinct from and unlike those of serotonergic psychedelics like LSD but similar to those of ibogaine.[5][6][7][8] Along with harmaline and tetrahydroharmine, harmine is one of the psychoactive constituents of Banisteriopsis caapi.[1][4] These other constituents, particularly harmaline, may be the more relevant hallucinogenic constituents of this plant.[1][4]
Monoamine oxidase inhibitor
Harmine is a reversible inhibitor of monoamine oxidase A (RIMA), a type of monoamine oxidase inhibitor (MAOI) as it reversibly inhibits monoamine oxidase A (MAO-A), but not monoamine oxidase B (MAO-B).[3] Doses of harmine that are active as a RIMA in combination with dimethyltryptamine (DMT) are in the range of 140 to 190 mg orally, whereas smaller doses in the range of 120 to 140 mg were ineffective.[4] However, its RIMA activity at the preceding effective doses was described as significant but modest.[4] Oral or intravenous harmine doses ranging from 30 to 300 mg may cause agitation, bradycardia or tachycardia, blurred vision, hypotension, and paresthesias.
Medically significant amounts of harmine occur in the plants Syrian rue and Banisteriopsis caapi. These plants also contain notable amounts of harmaline,[2] which is also a RIMA.[3] The psychoactive ayahuasca brew is made from B. caapi stem bark usually in combination with dimethyltryptamine (DMT) containing Psychotria viridis leaves. DMT is a psychedelic drug, but it is not orally active unless it is ingested with MAOIs. This makes harmine a vital component of the ayahuasca brew with regard to its ability to induce a psychedelic experience.[9] Syrian rue or synthetic harmine is sometimes used to substitute B. caapi in the oral use of DMT.[10]
Harmine was used or investigated as an antiparkinsonian medication since the late 1920s until the early 1950s. It was replaced by other medications.[11]
Other uses

Harmine is a useful fluorescent pH indicator. As the pH of its local environment increases, the fluorescence emission of harmine decreases.
Due to its MAO-A specific binding, carbon-11 labeled harmine can be used in positron emission tomography to study MAO-A dysregulation in several psychiatric and neurologic illnesses.[12]
Adverse effects
A 2024 Phase 1 clinical trial investigating pharmaceutical-grade harmine hydrochloride in healthy adults found that the maximum tolerated dose (MTD) is approximately 2.7 mg/kg body weight.[13]
Below this threshold, harmine is generally well-tolerated with minimal adverse effects. Above 2.7 mg/kg, common adverse effects include nausea and vomiting, which typically occur 60–90 minutes after ingestion. Other reported effects include drowsiness, dizziness, and impaired concentration. These effects are generally mild to moderate in severity and resolve within several hours.
No serious adverse cardiovascular effects were observed at any dose tested (up to 500 mg), though rare instances of transient hypotension occurred during episodes of vomiting. Unlike some traditional preparations containing harmine (such as Ayahuasca), pure harmine did not cause diarrhea in study participants.
The study found that adverse effects were more common in participants with lower body weight when given fixed doses, leading the researchers to conclude that 2.7 mg/kg represents a more useful threshold than fixed dosing.
Pharmacology
Pharmacodynamics
| Target | Affinity (Ki, nM) |
|---|---|
| 5-HT1A | >10,000 |
| 5-HT1B | ND |
| 5-HT1D | >10,000 (calf/pig) |
| 5-HT1E | ND |
| 5-HT1F | ND |
| 5-HT2A | 230–397 (rat) |
| 5-HT2B | ND |
| 5-HT2C | 5,340 (rat) |
| 5-HT3 | ND |
| 5-HT4 | ND |
| 5-HT5A | ND |
| 5-HT6 | ND |
| 5-HT7 | ND |
| α1A–α1D | ND |
| α2 | >10,000 (rat) |
| α2A–α2C | ND |
| β1–β3 | ND |
| D1 | ND |
| D2 | >10,000 |
| D3–D5 | ND |
| H1–H4 | ND |
| M1–M5 | ND |
| I1 | 629 (IC50) |
| I2 | 10 |
| σ1, σ2 | ND |
| MOR | >100,000 (bovine) |
| DOR | >100,000 (bovine) |
| DOR | >100,000 (bovine) |
| TAAR1 | ND |
| BDZ | >10,000 (rat) |
| PCP | ND |
| SERT | ND |
| NET | ND |
| DAT | 12,000 (IC50) |
| MAO-A | 1.0–16.9 (Ki) 1.0–380 (IC50) |
| MAO-B | 120,800 (Ki) ND (IC50) |
| DYRK1A | 12–700 (IC50) |
| Notes: The smaller the value, the more avidly the drug binds to the site. All proteins are human unless otherwise specified. Refs: [14][15][16][17][18][19][20][21][22] | |
The pharmacology of harmine has been studied.[23][20][16] It showed affinity (Ki) for the serotonin 5-HT2A receptor (Ki = 230–397 nM) and for the serotonin 5-HT2C receptor (Ki = 5,340 nM), but not for the serotonin 5-HT1A receptor, the dopamine D2 receptor, or the benzodiazepine site of the GABAA receptor (all Ki = >10,000 nM).[20][16][17] The drug showed among the highest affinity for the serotonin 5-HT2A receptor of any other β-carboline, with a few exceptions.[16][17] Its functional activity at the serotonin 5-HT2A receptor has not been studied, but harmine has been found to increase dopamine release in the nucleus accumbens in a serotonin 5-HT2A receptor-dependent manner as evidenced by reversal by ketanserin.[20][24] However, other β-carbolines like harmaline did not activate the serotonin 5-HT2A receptor even at very high concentrations in vitro.[25] Harmine has been found to be antagonistic to serotonin in certain tissues similarly to LSD.[26]
Harmine has also shown affinity for the imidazoline I2 receptor (Ki = 10 nM).[20] It has been suggested that this action might be involved in or responsible for its hallucinogenic effects.[20] The drug is a potent inhibitor of DYRK1A (Ki or IC50 = 33–700 nM) and a very weak dopamine reuptake inhibitor (IC50 = 12,000 nM).[20] Conversely, it is not a dopamine transporter (DAT) substrate or dopamine releasing agent.[20] Harmine is a highly potent inhibitor of monoamine oxidase A (MAO-A) (Ki = 16.9 nM, IC50 = 2.0–380 nM).[20][27][23] It shows 10,000-fold selectivity for MAO-A over monoamine oxidase B (MAO-B).[20] Unlike ibogaine and noribogaine, harmine does not bind to the κ-opioid receptor or other opioid receptors.[22]
In contrast to harmaline and 6-methoxyharmalan, which fully substituted for the psychedelic drug DOM in rodent drug discrimination tests, but similarly to harmane, harmine failed to significantly substitute for DOM and produced behavioral disruption at higher doses.[28]
Pharmacokinetics
The pharmacokinetics of harmine have been studied and described.[20][23] The plasma elimination half-life of harmine is on the order of 1 to 3 hours.[29]
Chemistry
Harmine, also known as 7-methoxy-1-methyl-β-carboline, is a substituted β-carboline and cyclized tryptamine derivative. Analogues of harmine include harmaline and tetrahydroharmine, among others. A positional isomer of harmine is 6-methoxyharman and analogues of that isomer include 6-methoxyharmalan and 6-methoxytetrahydroharmine (6-MeO-THH).
Natural occurrence
Harmine is found in a wide variety of different organisms, most of which are plants.
Alexander Shulgin lists about thirty different species known to contain harmine, including seven species of butterfly in the family Nymphalidae.[4]
The harmine-containing plants include tobacco, Peganum harmala, two species of passiflora, and numerous others. Lemon balm (Melissa officinalis) contains harmine.[30]
In addition to B. caapi, at least three members of the Malpighiaceae contain harmine, including two more Banisteriopsis species and the plant Callaeum antifebrile. Callaway, Brito and Neves (2005) found harmine levels of 0.31–8.43% in B. caapi samples.[31]
The family Zygophyllaceae, which P. harmala belongs to, contains at least two other harmine-bearing plants: Peganum nigellastrum and Zygophyllum fabago.
Biosynthesis
The coincident occurrence of β-carboline alkaloids and serotonin in Peganum harmala indicates the presence of two very similar, interrelated biosynthetic pathways, which makes it difficult to definitively identify whether free tryptamine or L-tryptophan is the precursor in the biosynthesis of harmine.[32] However, it is postulated that L-tryptophan is the most likely precursor, with tryptamine existing as an intermediate in the pathway.
The following figure shows the proposed biosynthetic scheme for harmine.[33] The Shikimate acid pathway yields the aromatic amino acid, L-tryptophan. Decarboxylation of L-tryptophan by aromatic L-amino acid decarboxylase (AADC) produces tryptamine (I), which contains a nucleophilic center at the C-2 carbon of the indole ring due to the adjacent nitrogen atom that enables the participation in a Mannich-type reaction. Rearrangements enable the formation of a Schiff base from tryptamine, which then reacts with pyruvate in II to form a β-carboline carboxylic acid. The β-carboline carboxylic acid subsequently undergoes decarboxylation to produce 1-methyl β-carboline III. Hydroxylation followed by methylation in IV yields harmaline. The order of O-methylation and hydroxylation have been shown to be inconsequential to the formation of the harmaline intermediate.[32] In the last step V, the oxidation of harmaline is accompanied by the loss of water and effectively generates harmine.
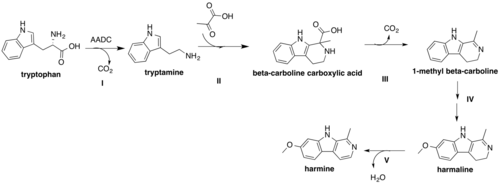
The difficulty distinguishing between L-tryptophan and free tryptamine as the precursor of harmine biosynthesis originates from the presence of the serotonin biosynthetic pathway, which closely resembles that of harmine, yet necessitates the availability of free tryptamine as its precursor.[32] As such, it is unclear if the decarboxylation of L-tryptophan, or the incorporation of pyruvate into the basic tryptamine structure is the first step of harmine biosynthesis. However, feeding experiments involving the feeding of one of tryptamine to hairy root cultures of P. harmala showed that the feeding of tryptamine yielded a great increase in serotonin levels with little to no effect on β-carboline levels, confirming that tryptamine is the precursor for serotonin, and indicating that it is likely only an intermediate in the biosynthesis of harmine; otherwise, comparable increases in harmine levels would have been observed.[33]
History
J. Fritzsche was the first to isolate and name harmine. He isolated it from the husks of Peganum harmala seeds in 1848. The related harmaline was already isolated and named by Fr. Göbel in 1837 from the same plant.[34][11] The pharmacology of harmine was not studied in detail until 1895.[11] The structures of harmine and harmaline were determined in 1927 by Richard Helmuth Fredrick Manske and colleagues.[35][36]
In 1905, the Colombian naturalist and chemist, Rafael Zerda-Bayón suggested the name telepathine to the then unknown hallucinogenic ingredient in ayahuasca brew.[2][11] "Telepathine" comes from "telepathy", as Zerda-Bayón believed that ayahuasca induced telepathic visions.[2][37] In 1923, the Colombian chemist, Guillermo Fischer-Cárdenas was the first to isolate harmine from Banisteriopsis caapi, which is an important herbal component of ayahuasca brew. He called the isolated harmine "telepathine".[2] This was solely to honor Zerda-Bayón, as Fischer-Cárdenas found that telepathine had only mild non-hallucinogenic effects in humans.[38] In 1925, Barriga Villalba, professor of chemistry at the University of Bogotá, isolated harmine from B. caapi, but named it "yajéine",[11] which in some texts is written as "yageine".[2] In 1927, F. Elger, who was a chemist working at Hoffmann-La Roche, isolated harmine from B. caapi. With the assistance of Professor Robert Robinson in Manchester, Elger showed that harmine (which was already isolated in 1848) was identical with telepathine and yajéine.[39][11] In 1928, Louis Lewin isolated harmine from B. caapi, and named it "banisterine",[40] but this supposedly novel compound was soon also shown to be harmine.[11] Lewin, in 1928, was the first to describe the subjective effects of harmine in the literature.[1]
Harmine was first patented by Jialin Wu and others who invented ways to produce new harmine derivatives with enhanced antitumor activity and lower toxicity to human nervous cells.[41]
Society and culture
Names
Harmine is the most common name of the compound.[42] It is also known by other names including banisterine, banisterin, telepathine, telopathin, leucoharmine, yagin, and yageine, among others.[42][43][2]
Legal status
Australia
Harmala alkaloids are considered Schedule 9 prohibited substances under the Poisons Standard (October 2015).[44] A Schedule 9 substance is a substance which may be abused or misused, the manufacture, possession, sale or use of which should be prohibited by law except when required for medical or scientific research, or for analytical, teaching or training purposes with approval of Commonwealth and/or State or Territory Health Authorities.[44]
Exceptions are made when in herbs, or preparations, for therapeutic use such as: (a) containing 0.1 per cent or less of harmala alkaloids; or (b) in divided preparations containing 2 mg or less of harmala alkaloids per recommended daily dose.[44]
Research
Pancreatic islet cell proliferation
Harmine is currently the only known drug that induces proliferation (rapid mitosis and subsequent mass growth) of pancreatic alpha (α) and beta (β) cells in adult humans.[45] These islet sub-cells are normally resistant to growth stimulation in the adult stage of a human's life, as the cell mass plateaus at around age 10 and remains virtually unchanged.
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Indolealkylamines and Related Compounds". Hallucinogenic Agents. Bristol: Wright-Scientechnica. 1975. pp. 98–144. ISBN 978-0-85608-011-1. OCLC 2176880.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Banisteriopsis caapi, a Forgotten Potential Therapy for Parkinson's Disease?". Movement Disorders Clinical Practice 3 (1): 19–26. 2015. doi:10.1002/mdc3.12242. PMID 30713897.
- ↑ 3.0 3.1 3.2 "The Therapeutic Potentials of Ayahuasca: Possible Effects against Various Diseases of Civilization". Frontiers in Pharmacology 7: 35. 2016. doi:10.3389/fphar.2016.00035. PMID 26973523.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 TiHKAL: The Continuation. Transform Press. 1997. pp. 713–714. ISBN 978-0-9630096-9-2.
- ↑ "Ibogaine: Fantasy and Reality". The Healing Journey: New Approaches to Consciousness. New York: Parthenon Books. 1973. pp. 174–228. ISBN 978-0-394-48826-4. https://www.claudionaranjo.net/pdf_files/psychedelics/healing_journey_ch_5_ibogaine_english.pdf.
- ↑ "Psycotherapeutic Possibilities of New Fantasy-Enhancing Drugs". Clinical Toxicology 2 (2): 209–224. 1969. doi:10.3109/15563656908990930. ISSN 0009-9309. http://www.tandfonline.com/doi/full/10.3109/15563656908990930. Retrieved 27 May 2025.
- ↑ "Ibogaine Acute Administration in Rats Promotes Wakefulness, Long-Lasting REM Sleep Suppression, and a Distinctive Motor Profile". Frontiers in Pharmacology 9. 2018. doi:10.3389/fphar.2018.00374. PMID 29755349.
- ↑ "Chapter 4 Drug discrimination studies with ibogaine". The Alkaloids: Chemistry and Biology. 56. Elsevier. 2001. pp. 63–77. doi:10.1016/s0099-9598(01)56008-3. ISBN 978-0-12-469556-6. https://www.iceers.org/Documents_ICEERS_site/Scientific_Papers/ibogaine/Ibogaine%20Proceedings/ch04_Discrimination_Helsley.pdf.
- ↑ "Ayahuasca: Psychological and Physiologic Effects, Pharmacology and Potential Uses in Addiction and Mental Illness". Current Neuropharmacology 17 (2): 108–128. 2019. doi:10.2174/1570159X16666180125095902. PMID 29366418.
- ↑ "Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review". Medicines (Basel, Switzerland) 6 (4): 106. October 2019. doi:10.3390/medicines6040106. PMID 31635364.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 Foley PB (2001). "V. Encephalitis lethargica: New strategies in the therapy of parkinsonism". Beans, roots and leaves: a brief history of the pharmacological therapy of parkinsonism (PhD thesis). Bavarian Julius Maximilian University. pp. 166–180. Retrieved 2020-11-22.
- ↑ "Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain". Journal of Cerebral Blood Flow and Metabolism 26 (3): 330–344. March 2006. doi:10.1038/sj.jcbfm.9600197. PMID 16079787.
- ↑ "A Phase 1 single ascending dose study of pure oral harmine in healthy volunteers". Journal of Psychopharmacology (Oxford, England) 38 (10): 911–923. October 2024. doi:10.1177/02698811241273772. PMID 39301926.
- ↑ "Kᵢ Database". 18 June 2025. https://pdspdb.unc.edu/kidb2/kidb/web/kis-results/index?KisResultsSearch%5Binput_receptors%5D=&KisResultsSearch%5Binput_sources%5D=&KisResultsSearch%5Binput_species%5D=&KisResultsSearch%5Binput_hot_ligands%5D=&KisResultsSearch%5Binput_test_ligands%5D=&KisResultsSearch%5Binput_test_ligands%5D%5B%5D=919&KisResultsSearch%5Binput_citations%5D=&KisResultsSearch%5BsearchType%5D=&KisResultsSearch%5Bki_val_from%5D=&KisResultsSearch%5Bki_val_to%5D=&KisResultsSearch%5Bcustom_ki_val%5D=.
- ↑ "BindingDB BDBM100152 7-methoxy-1-methyl-9H-beta-carboline;hydrochloride::7-methoxy-1-methyl-9H-pyrido[3,4-bindole;hydrochloride::HARMINE::Harmine hydrochloride::MLS002153910::SMR001233259::cid_5359389"]. https://www.bindingdb.org/rwd/bind/chemsearch/marvin/MolStructure.jsp?monomerid=100152.
- ↑ 16.0 16.1 16.2 16.3 "Binding of beta-carbolines and related agents at serotonin (5-HT(2) and 5-HT(1A)), dopamine (D(2)) and benzodiazepine receptors". Drug and Alcohol Dependence 60 (2): 121–132. August 2000. doi:10.1016/s0376-8716(99)00148-9. PMID 10940539.
- ↑ 17.0 17.1 17.2 "Investigation of hallucinogenic and related beta-carbolines". Drug and Alcohol Dependence 50 (2): 99–107. April 1998. doi:10.1016/s0376-8716(97)00163-4. PMID 9649961.
- ↑ "beta-carboline binding to imidazoline receptors". Drug and Alcohol Dependence 64 (2): 203–208. October 2001. doi:10.1016/s0376-8716(01)00123-5. PMID 11543990.
- ↑ "Synthesis and evaluation of β-carboline derivatives as potential monoamine oxidase inhibitors". Bioorganic & Medicinal Chemistry 19 (1): 134–144. January 2011. doi:10.1016/j.bmc.2010.11.041. PMID 21183355.
- ↑ 20.00 20.01 20.02 20.03 20.04 20.05 20.06 20.07 20.08 20.09 20.10 "Developments in harmine pharmacology--implications for ayahuasca use and drug-dependence treatment". Progress in Neuro-Psychopharmacology & Biological Psychiatry 39 (2): 263–272. December 2012. doi:10.1016/j.pnpbp.2012.06.001. PMID 22691716.
- ↑ "β-Carboline as a Privileged Scaffold for Multitarget Strategies in Alzheimer's Disease Therapy". Journal of Medicinal Chemistry 64 (3): 1392–1422. February 2021. doi:10.1021/acs.jmedchem.0c01887. PMID 33528252.
- ↑ 22.0 22.1 "Mechanisms of action of ibogaine and harmaline congeners based on radioligand binding studies". Brain Research 571 (2): 242–247. February 1992. doi:10.1016/0006-8993(92)90661-r. PMID 1377086.
- ↑ 23.0 23.1 23.2 "Pharmacological effects of harmine and its derivatives: a review". Archives of Pharmacal Research 43 (12): 1259–1275. December 2020. doi:10.1007/s12272-020-01283-6. PMID 33206346.
- ↑ "Harmine augments electrically evoked dopamine efflux in the nucleus accumbens shell". Journal of Psychopharmacology (Oxford, England) 27 (1): 98–108. January 2013. doi:10.1177/0269881112463125. PMID 23076833.
- ↑ "Binding of beta-carbolines at 5-HT(2) serotonin receptors". Bioorganic & Medicinal Chemistry Letters 13 (24): 4421–4425. December 2003. doi:10.1016/j.bmcl.2003.09.027. PMID 14643338. "[...] several β-carbolines, including harmaline (1) and its positional isomer 6-methoxyharmalan (4) substituted for the hallucinogenic (5-HT2A agonist) phenylalkylamine [DOM] in a drug discrimination task with rats trained to discriminate DOM from saline vehicle.10 However, neither harmaline (1; Ki=7790 nM) nor 6-methoxyharmalan (4; Ki=5600 nM) binds with high affinity at 5-HT2A receptors, and both were found to lack action as 5-HT2A agonists in a phosphoinositol (PI) hydrolysis assay.5,9 [...] At this time, it is not known if the actions of 1 and 4 in the PI hydrolysis assay reflect their low affinity, low efficacy, or whether the actions of the β-carbolines (in drug discrimination and/or other assays) is attributable to, or compromised by, their actions at other populations of receptors—particularly 5-HT receptors—or by possible interactions with the serotonin transporter.".
- ↑ "Pharmacology and Classification of LSD-like Hallucinogens". Drug Addiction II: Amphetamine, Psychotogen, and Marihuana Dependence. Berlin, Heidelberg: Springer Berlin Heidelberg. 1977. pp. 305–368. doi:10.1007/978-3-642-66709-1_3. ISBN 978-3-642-66711-4. https://books.google.com/books?id=gb_uCAAAQBAJ&pg=PA305. "WOOLLEY and SHAW (1954a) also found that LSD as well as yohimbine and harmine antagonized the effects of 5-HT on aortic rings and rat uterus."
- ↑ "Pharmacology and Structure-Activity Relationship of Natural Products With Psychoactive Effects From Salvia divinorum, Mitragyna speciosa, and Ayahuasca". Studies in Natural Products Chemistry. 53. Elsevier. 2017. pp. 1–44. doi:10.1016/b978-0-444-63930-1.00001-6. ISBN 978-0-444-63930-1. https://linkinghub.elsevier.com/retrieve/pii/B9780444639301000016. Retrieved 18 June 2025.
- ↑ "DOM-stimulus generalization to LSD and other hallucinogenic indolealkylamines". European Journal of Pharmacology 86 (3–4): 453–459. January 1983. doi:10.1016/0014-2999(83)90196-6. PMID 6572591.
- ↑ Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. 2008. pp. 727–728.
- ↑ "Harmala Alkaloids as Bee Signaling Chemicals". Journal of Student Research 1 (1): 23–32. 2012. doi:10.47611/jsr.v1i1.30. http://www.jofsr.com/index.php/path/article/view/30.
- ↑ "Phytochemical analyses of Banisteriopsis caapi and Psychotria viridis". Journal of Psychoactive Drugs 37 (2): 145–150. June 2005. doi:10.1080/02791072.2005.10399795. PMID 16149327.
- ↑ 32.0 32.1 32.2 "Biosynthesis of Serotonin and Beta-carboline Alkaloids in Hairy Root Cultures of Peganum Harmala". Phytochemistry 33 (3): 593–597. 1993. doi:10.1016/0031-9422(93)85453-x. Bibcode: 1993PChem..33..593B.
- ↑ 33.0 33.1 "Limitations of Feeding Experiments in Studying Alkaloid Biosynthesis in Peganum Harmala Callus Cultures". Phytochemistry 13 (4): 735–742. 1974. doi:10.1016/s0031-9422(00)91406-7. Bibcode: 1974PChem..13..735N.
- ↑ "Bestandtheile der Samen von Peganum Harmala". Justus Liebigs Annalen der Chemie 64 (3): 360–369. 1848. doi:10.1002/jlac.18480640353. https://chemistry-europe.onlinelibrary.wiley.com/doi/abs/10.1002/jlac.18480640353.
- ↑ "Harmine and harmaline. Part IX. A synthesis of harmaline". Journal of the Chemical Society: 1–14. 1927. doi:10.1039/JR9270000001. https://pubs.rsc.org/en/content/articlelanding/1927/jr/jr9270000001.
- ↑ , Howard S."Method of treating chemical dependency using β-carboline alkaloids, derivatives and salts thereof" US patent, published 1997-01-07
- ↑ "Telepathy and Telepathine". American Druggist 68 (4): 15. 1920. https://www.samorini.it/doc1/alt_aut/ad/baldo-telepathy-and-telepathin.pdf.
- ↑ Fischer-Cárdenas G (1923). "V. Encephalitis lethargica: New strategies in the therapy of parkinsonism" (PDF). Estudio sobre el principio activo del Yagé (PhD). Universidad Nacional. Retrieved 2020-11-22.
- ↑ "Über das Vorkommen von Harmin in einer südamerikanischen Liane (Yagé)". Helvetica Chimica Acta 11 (1): 162–166. 1928. doi:10.1002/hlca.19280110113. Bibcode: 1928HChAc..11..162E. https://onlinelibrary.wiley.com/doi/abs/10.1002/hlca.19280110113.
- ↑ "The beta-carboline hallucinogens of South America". Journal of Psychoactive Drugs 14 (3): 205–220. 1982. doi:10.1080/02791072.1982.10471930. PMID 6754896.
- ↑ Wu J, Chen R, Cao F, Yu Z, Wang W, Peng, "Harmine derivatives, intermediates used in their preparations, preparation processes and use thereof", EP patent, published 2006-03-15
- ↑ 42.0 42.1 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA139. Retrieved 14 October 2025.
- ↑ "The simple β-carboline alkaloids". Phytochemistry 19 (8): 1573–1582. 1980. doi:10.1016/S0031-9422(00)83773-5. Bibcode: 1980PChem..19.1573A. http://www.sciencedirect.com/science/article/pii/S0031942200837735.
- ↑ 44.0 44.1 44.2 Poisons Standard October 2015 https://www.comlaw.gov.au/Details/F2015L01534
- ↑ "A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication". Nature Medicine 21 (4): 383–388. April 2015. doi:10.1038/nm.3820. PMID 25751815.
External links
- Harmine - Isomer Design
- Harmine - PsychonautWiki
- Harmala Alkaloids - Erowid
- Harmine - TiHKAL - Erowid
- Harmine - TiHKAL - Isomer Design
 |
