Chemistry:Hexafluorophosphazene
| |
| Names | |
|---|---|
| IUPAC name
2,2,4,4,6,6-Hexafluoro-1,3,5,2λ5,4λ5,6λ5-triazatriphosphinine
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| (NPF 2) 3 | |
| Molar mass | 248.933 g·mol−1 |
| Appearance | White powder or lumps[1] |
| Melting point | 27 °C (81 °F; 300 K) |
| Boiling point | 51 °C (124 °F; 324 K) |
| decomposes | |
| Solubility | Toluene[1] |
| Structure | |
| Planar P 3N 3 ring | |
| 0 D | |
| Hazards | |
| Main hazards | Corrosive |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| PP260Script error: No such module "Preview warning".Category:GHS errors, PP264Script error: No such module "Preview warning".Category:GHS errors, PP280Script error: No such module "Preview warning".Category:GHS errors, PP303 + P361 + P353Script error: No such module "Preview warning".Category:GHS errors, PP301 + P330 + P331Script error: No such module "Preview warning".Category:GHS errors, PP363Script error: No such module "Preview warning".Category:GHS errors, PP304 + P340 + P310Script error: No such module "Preview warning".Category:GHS errors, PP305 + P351 + P338 + P310Script error: No such module "Preview warning".Category:GHS errors, PP405Script error: No such module "Preview warning".Category:GHS errors, PP501Script error: No such module "Preview warning".Category:GHS errors | |
| Related compounds | |
Related compounds
|
Hexachlorophosphazene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
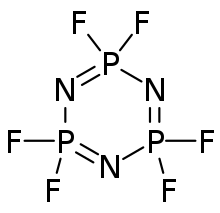
Hexafluorophosphazene is an inorganic compound with the formula (NPF
2)
3. It takes the form of a white powder or lumps. It is sensitive to moisture and heat.[1]
Structure
The molecule has a cyclic, unsaturated backbone consisting of alternating phosphorus and nitrogen centers, and can be viewed as a trimer of the hypothetical compound N≡PF
2. Its classification as a phosphazene highlights its relationship to benzene. Hexafluorophosphazene has a hexagonal P
3N
3 ring with six equivalent P–N bonds. Each phosphorus atom is additionally bonded to two fluorine atoms.[citation needed]
The molecule possesses D3h symmetry, and each phosphorus center is tetrahedral.
The P
3N
3 ring in hexachlorophosphazene deviates from planarity and is slightly ruffled (see chair conformation). By contrast, the P
3N
3 ring in hexafluorophosphazene is completely planar.
References
 |

