Chemistry:Hexaphenylcarbodiphosphorane
| |
| Names | |
|---|---|
| Preferred IUPAC name
Methanediylidenebis(triphenyl-λ5-phosphane) | |
| Other names
bis(triphenylphosphoranylidene)methane
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C37H30P2 | |
| Molar mass | 536.595 g·mol−1 |
| Appearance | yellow solid |
| Density | 1.205 g/cm3 |
| Melting point | 198–201 °C (388–394 °F; 471–474 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
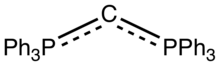
Hexaphenylcarbodiphosphorane is the organophosphorus compound with the formula C(PPh3)2 (where Ph = C6H5). It is a yellow, moisture-sensitive solid. The compound is classified as an ylide and as such carries significant negative charge on carbon. It is isoelectronic with bis(triphenylphosphine)iminium. The P-C-P angle is 131°.[1] The compound has attracted attention as an unusual ligand in organometallic chemistry.[2]
The pure compound has two crystalline phases: a metastable monoclinic C2 phase that is triboluminescent, and an orthorhombic P222 form that is not. Both polymorphs are photoluminescent, with respective peak wavelengths at 540 and 575 nm.[3]
Preparation
The compound was originally prepared by deprotonation of the phosphonium salt [HC(PPh3)2]Br using potassium.[4]
An improved procedure entails production of the same double phosphonium salt from methylene bromide. The double deprotonation is effected with potassium amide.[5]
Related compounds
- Methylenetriphenylphosphorane (CH2=PPh3), the parent Wittig reagent
References
- ↑ Tonner, Ralf; Oexler, Florian; Neumueller, Bernhard; Petz, Wolfgang; Frenking, Gernot (2006). "Carbodiphosphoranes: The Chemistry of Divalent Carbon(0)". Angewandte Chemie International Edition 45 (47): 8038–8042. doi:10.1002/anie.200602552. PMID 17075933.
- ↑ Petz, W.; Frenking, G. (2010). "Carbodiphosphoranes and Related Ligands". Transition Metal Complexes of Neutral eta1-Carbon Ligands. Topics in Organometallic Chemistry. 30. 49–92. doi:10.1007/978-3-642-04722-0_3. ISBN 978-3-642-04721-3. Bibcode: 2010tmcn.book...49P.
- ↑ Hardy, Gordon E.; Kaska, William C.; Chandra, B. P.; Zink, Jeffrey I. (March 1981). "Triboluminescence-structure relationships in polymorphs of hexaphenylcarbodiphosphorane and anthranilic acid, molecular crystals, and salts". Journal of the American Chemical Society 103 (5): 1074–1079. doi:10.1021/ja00395a014.
- ↑ Ramirez, Fausto; Desai, N. B.; Hansen, B.; McKelvie, N. (1961). "Hexaphenylcarbodiphosphorane, (C6H5)3P:C:P(C6H5)3". Journal of the American Chemical Society 83 (16): 3539–40. doi:10.1021/ja01477a052.
- ↑ Gruber, Marco; Bauer, Walter; Maid, Harald; Schöll, Kilian; Tykwinski, Rik R. (2017). "Synthetic and NMR Studies on Hexaphenylcarbodiphosphorane (Ph3P=C=PPh3)". Inorganica Chimica Acta 468: 152–158. doi:10.1016/j.ica.2017.04.018.
 |

