Chemistry:Bis(triphenylphosphine)iminium chloride
| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexaphenyl-1λ5-diphosphaz-1-en-3-ium chloride | |
| Other names
PNP chloride
PPN chloride Bis(triphenylphosphine)iminium chloride Bis(triphenylphosphoranylidene)iminium chloride Bis(triphenylphosphoranylidene)ammonium chloride Hexaphenyldiphosphazenium chloride Selectophore | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| [((C 6H 5) 3P) 2N]Cl | |
| Molar mass | 574.03 g/mol |
| Appearance | colourless solid |
| Melting point | 260 to 262 °C (500 to 504 °F; 533 to 535 K) |
| moderate | |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H332, H335 | |
| P261, P264, P271, P280, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Related compounds | |
Related compounds
|
Tetraphenylarsonium chloride Tetrabutylammonium chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bis(triphenylphosphine)iminium chloride is the chemical compound with the formula [((C
6H
5)
3P)
2N]Cl, often abbreviated [(Ph
3P)
2N]Cl, where Ph is phenyl C
6H
5, or even abbreviated [PPN]Cl or [PNP]Cl or PPNCl or PNPCl, where PPN or PNP stands for (Ph
3P)
2N. This colorless salt is a source of the [(Ph
3P)
2N]+
cation (abbreviated PPN+
or PNP+
), which is used as an unreactive and weakly coordinating cation to isolate reactive anions. [(Ph
3P)
2N]+
is a phosphazene.
Synthesis and structure
[(Ph
3P)
2N]Cl is prepared in two steps from triphenylphosphine Ph
3P:[1]
- Ph
3P + Cl
2 → Ph
3PCl
2
This triphenylphosphine dichloride Ph
3PCl
2 is related to phosphorus pentachloride PCl
5. Treatment of this species with hydroxylamine in the presence of Ph
3P results in replacement of the two single P–Cl bonds in Ph
3PCl
2 by one double P=N bond:
- 2 Ph
3PCl
2 + NH
2OH · HCl + Ph
3P → [(Ph
3P)
2N]Cl + 4HCl + Ph
3PO
Triphenylphosphine oxide Ph
3PO is a by-product.
Bis(triphenylphosphine)iminium chloride is described as [(Ph
3P)
2N]+
Cl−
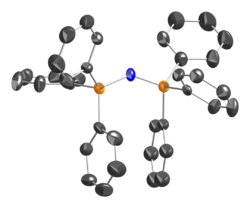
. The structure of the bis(triphenylphosphine)iminium cation [(Ph
3P)
2N]+
is [Ph
3P=N=PPh
3]+
. The P=N=P angle in the cation is flexible, ranging from ~130 to 180° depending on the salt. Bent and linear forms of the P=N=P connections have been observed in the same unit cell.[2] The same shallow potential well for bending is observed in the isoelectronic species bis(triphenylphosphoranylidene)methane, Ph
3P=C=PPH
3, as well as the more distantly related molecule carbon suboxide, O=C=C=C=O. For the solvent-free chloride salt [(Ph
3P)
2N]Cl, the P=N=P bond angle was determined to be 133°.[3] The two P=N bonds are equivalent, and their length is 1.597(2) Å.
Use as reagent
In the laboratory, [(Ph
3P)
2N]Cl is the main precursor to [(Ph
3P)
2N]+
salts. Using salt metathesis reactions, nitrite, azide, and other small inorganic anions can be obtained with [(Ph
3P)
2N]+
cations. The resulting salts [(Ph
3P)
2N]+
NO−
2, [(Ph
3P)
2N]+
N−
3, etc. are soluble in polar organic solvents.
[(Ph
3P)
2N]+
forms crystalline salts with a range of anions that are otherwise difficult to crystallize. Its effectiveness is partially attributable to its rigidity, reflecting the presence of six phenyl rings. Often [(Ph
3P)
2N]+
forms salts that are more air-stable than salts with smaller cations such as those containing quaternary ammonium cation [NR
4]+
, or alkali metal cations. This effect is attributed to the steric shielding provided by this voluminous cation. Illustrative [(Ph
3P)
2N]+
salts of reactive anions include [(Ph
3P)
2N]+
[HFe(CO)
4]−
, [(Ph
3P)
2N]+
[Co(CO)
4]−
, ([(Ph
3P)
2N]+
)
2[M
2(CO)
10]2+ (M = Cr, Mo, W), and [(Ph
3P)
2N]+
[Fe(CO)
3(NO)]−
.[1] The role of ion pairing in chemical reactions is often clarified by examination of the related salt derived from [(Ph
3P)
2N]+
.
Related cations
A phosphazenium cation related to [(Ph
3P)
2N]+
is [(((CH
3)
2N)
3P)
2N]+
.[4]
References
- ↑ 1.0 1.1 Ruff, J.K.; Schlientz, W.J. (1974). "μ‐Nitridobis(triphenylphosphorus)(l+) ("PPN") Salts with Metal Carbonyl Anions". Inorganic Syntheses. 15. 84–90. doi:10.1002/9780470132463.ch19. ISBN 9780470132463.
- ↑ Hardy, Gordon E.; Zink, Jeffrey I.; Kaska, W. C.; Baldwin, J. C. (December 1978). "Structure and triboluminescence of polymorphs of hexaphenylcarbodiphosphorane". Journal of the American Chemical Society 100 (25): 8001–8002. doi:10.1021/ja00493a035.
- ↑ "Solvate-free bis-(triphenylphosphine)iminium chloride". Acta Crystallographica Section E 66 (Pt 12): o3185. November 2010. doi:10.1107/S1600536810046325. PMID 21589480.
- ↑ Schwesinger, Reinhard (2001). "1,1,1,3,3,3-Hexakis(dimethylamino)-1λ5,3λ5-diphosphazenium fluoride". e-EROS Encyclopedia of Reagents for Organic Synthesis. pp. 1–2. doi:10.1002/047084289X.rh014m. ISBN 0471936235.
 |
