Chemistry:Hydrocinnamaldehyde
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Phenylpropanal | |
| Other names
3-phenylpropional, 3-phenylpropionaldehyde/ß-Phenylpropionaldehyde
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10O | |
| Molar mass | 134.178 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.018 g/cm3 |
| Melting point | −42 °C (−44 °F; 231 K) |
| Boiling point | 224 °C (435 °F; 497 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319 | |
| P264, P280, P302+352, P305+351+338, P321, P332+313, P337+313, P362 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
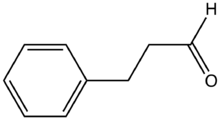
Hydrocinnamaldehyde is the organic compound with the formula C6H5CH2CH2CHO. It is produced by the hydrogenation of cinnamaldehyde. The compound is used in many mechanistic studies.[1] It is a common substrate in organic synthesis.[2][3]
References
- ↑ Enache, Dan I.; Edwards, Jennifer K.; Landon, Philip; Solsona-Espriu, Benjamin; Carley, Albert F.; Herzing, Andrew A.; Watanabe, Masashi; Kiely, Christopher J. et al. (2006). "Solvent-Free Oxidation of Primary Alcohols to Aldehydes Using Au-Pd/TiO2 Catalysts". Science 311 (5759): 362–365. doi:10.1126/science.1120560. PMID 16424335. Bibcode: 2006Sci...311..362E.
- ↑ Sasai, Hiroaki; Watanabe, Shizue; Suzuki, Takeyuki; Shibasaki, Masakatsu (2002). "Catalytic Asymmetric Synthesis of Nitroaldols Using a Lanthanum-Lithium-Binol Complex: (2S,3S)-2-Nitro-5-Phenyl-1,3-Pentanediol". Organic Syntheses 78: 14. doi:10.15227/orgsyn.078.0014.
- ↑ Abbott, Jason; Allais, Christophe; Roush, William R. (2015). "Enantioselective Reductive Syn-Aldol Reactions of 4-Acryloylmorpholine: Preparation of (2R, 3S)-3-Hydroxy-2-methyl-1-morpholino-5-phenylpentan-1-one". Organic Syntheses 92: 38–57. doi:10.15227/orgsyn.092.0038.
 |
