Chemistry:Iridium(III) bromide
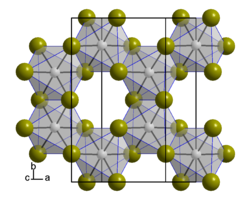 Crystal structure
| |
| Identifiers | |
|---|---|
| |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| Br3Ir | |
| Molar mass | 431.929 g·mol−1 |
| Appearance | dark reddish-brown solid[1] |
| Density | 6.82 g·cm−3[2] |
| insoluble[1] | |
| Solubility | insoluble in acids and bases[1] |
| Related compounds | |
Other anions
|
Iridium(III) hydroxide Iridium(III) chloride Iridium(III) iodide |
Other cations
|
Ruthenium(III) bromide Rhodium(III) bromide Osmium(III) bromide Platinum(III) bromide |
Related compounds
|
Iridium(II) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Iridium(III) bromide is a bromide of iridium(III), with the chemical formula of IrBr3.
Preparation
Iridium(III) bromide can be formed by reacting iridium(II) bromide and bromine. Its tetrahydrate can be formed by reacting iridium dioxide dihydrate with hydrobromic acid.[1] It can also be formed by the direct reaction of iridium and bromine at 8 atm and 570 °C.[3]
Properties
Iridium(III) bromide is a dark reddish-brown solid that is insoluble soluble in water, acids, and alkalis and decomposes to iridium(II) bromide on heating.[1] It crystallizes in a highly disordered layered structure of aluminum(III) chloride or chromium(III) chloride type, where the monoclinic unit cell contains four formula units. As with rhenium(III) chloride, rhenium(III) bromide, α-iridium(III) chloride and α-ruthenium(III) chloride, the disorder is due to the different stacking of the metal layers.[4] The light olive green tetrahydrate is slightly soluble in water but insoluble in ethanol and ether. When heated to 100 °C, it turns dark brown with release of water and decomposes to iridium and bromine at higher temperatures.[1] It reacts with germanium dibromide in hydrobromic acid solution to form a compound containing Ir-Ge bond, and adding Cs+ to it can separate Cs3[Ir(GeBr3)nBr6−n] (n=1, 2, 3).[5]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Kandiner, H. J. (2013-09-03) (in de). Iridium. Springer-Verlag. ISBN 978-3-662-12128-3. https://books.google.com/books?id=mImABwAAQBAJ.
- ↑ Perry, Dale L. (2016-04-19) (in en). Handbook of Inorganic Compounds. CRC Press. ISBN 978-1-4398-1462-8. https://books.google.com/books?id=SFD30BvPBhoC&pg=PA213.
- ↑ Livingstone, Stanley E. (2017-01-31) (in en). The Chemistry of Ruthenium, Rhodium, Palladium, Osmium, Iridium and Platinum: Pergamon Texts in Inorganic Chemistry, Volume 25. Elsevier. ISBN 978-1-4831-5840-2. https://books.google.com/books?id=_to_DQAAQBAJ&pg=PA1257.
- ↑ Brodersen, K.; Thiele, G.; Ohnsorge, H.; Recke, I.; Moers, F. (1968-07-01). "Die struktur des IrBr3 und über die ursachen der fehlordnungserscheinungen bei den in schichtenstrukturen kristallisierenden edelmetalltrihalogeniden" (in de). Journal of the Less Common Metals 15 (3): 347–354. doi:10.1016/0022-5088(68)90194-X. ISSN 0022-5088. https://dx.doi.org/10.1016/0022-5088%2868%2990194-X.
- ↑ Antonov, P. G.; Agapov, I. A.; Manasevich, D. S. Iridium(III) complexation with germanium(II) compounds in aqueous hydrobromic acid solutions (in Russian). Zhurnal Prikladnoi Khimii (Sankt-Peterburg), 1999. 72 (4): 556-559. ISSN: 0044-4618.
 |

