Chemistry:Isobornyl cyclohexanol
From HandWiki
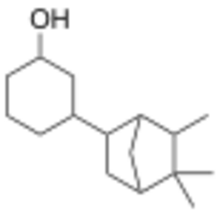
| |
| Names | |
|---|---|
| IUPAC name
3-(5,5,6-Trimethylbicyclo[2.2.1]heptan-2-yl)cyclohexanol
| |
| Other names
Isocamphyl cyclohexanol; 3-[5,5,6-Trimethylbicyclo[2.2.1]hept-2-yl]cyclohexan-1-ol; Sandal hexanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | IBCH |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| C16H28O | |
| Molar mass | 236.399 g·mol−1 |
| Appearance | Colorless to pale yellow clear viscous liquid[1] |
| Density | 0.97 g/mL[2] |
| Boiling point | 302 °C (576 °F; 575 K)[2] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | warning |
| Flash point | 110 °C (230 °F; 383 K)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Isobornyl cyclohexanol (IBCH, Sandenol,Sandela) is an organic compound used primarily as a fragrance because of its aroma which is similar to sandalwood oil. Its chemical structure is closely related to that of both α-Santalol and β-Santalol,[4] which are the primary constituents of sandalwood oil.
Sandalwood trees are endangered due to overharvesting,[5] leading to a high cost for the natural oil. IBCH is therefore produced as an economical alternative to the natural product.
References
- ↑ Sandal hexanol at thegoodscentscompany.com
- ↑ 2.0 2.1 2.2 3-(5,5,6-Trimethylbicyclo(2.2.1)hept-2-yl)cyclohexan-1-ol at Sigma-Aldrich
- ↑ GHS: PubChem 103005
- ↑ Demole, Edouard (1964). "Synthesis and relations between chemical constitution and odor in the 3-terpenylcyclohexanol series". Helvetica Chimica Acta 47 (7): 1766–74. doi:10.1002/hlca.19640470713.
- ↑ Jean-Francois Tremblay (2011). "Rhodia Invests in Synthetic Sandalwood". Chemical & Engineering News 89 (12): 24–25. doi:10.1021/CEN031511180238.
 |

