Chemistry:K252a
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
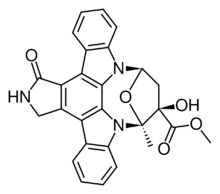
Methyl (13S,14R,16R)-14-hydroxy-13-methyl-5-oxo-6,7,13,14,15,16-hexahydro-5H-13,16-epoxydiindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-14-carboxylate | |
| Identifiers | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| Properties[1] | |
| C27H21N3O5 | |
| Molar mass | 467.481 g·mol−1 |
| Solubility in other solvents | Soluble in DMSO, dichloromethane, and methanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
K252a is an alkaloid isolated from Nocardiopsis bacteria. This staurosporine analog is a highly potent cell permeable inhibitor of CaM kinase and phosphorylase kinase (IC50 = 1.8 and 1.7 nmol/L, respectively). At higher concentrations it is also an efficient inhibitor of serine/threonine protein kinases (IC50 of 10 to 30 nmol/L).[2][3][4][5][6][7][8][9]
K252a is reported to promote myogenic differentiation in C2 mouse myoblasts[6] and has been shown to block the neuronal differentiation of rat pheochromocytoma PC12 cells by inhibition of trk tyrosine kinase activity.[10]
K252a has been reported in preclinical research as a potential treatment for psoriasis[11]
K252a inhibits tyrosine phosphorylation of Trk A induced by NGF. PC12 cells were incubated in the presence or absence of 10 ng/ml NGF with or without various concentrations of K252a.
See also
References
- ↑ K252a from Fermentek
- ↑ Ruegg, U.T. et al. (1989) Tips 10, 218.
- ↑ Eliot, L.H. et al. (1990) B.B.R.C. 171, 148.
- ↑ Simpson, D.l. et al. (1991) J. Neurosci. Res, 28, 148.
- ↑ Chin, L.S. et al. (1999) Cancer Invest. 17, 391.
- ↑ 6.0 6.1 Tapley, P. et al. (1992) Oncogene 7, 371.
- ↑ Hashimoto, S. (1998) J. Cell Biol. 107, 1531.
- ↑ Kase, H. et al. (1987) B.B.R.C. 142, 436.
- ↑ Hirayama E. et al. (2001) B.B.R.C. 285, 1237.
- ↑ Borasio, G.D. Neurosci. Lett. (1990) 108, 207.
- ↑ Promising New Treatments for Psoriasis, Sarah Dubois Declercq and Roxane Pouliot >.
The Scientific World Journal; Volume 2013, Article ID 980419; https://dx.doi.org/10.1155/2013/980419
Further reading
- "Total synthesis of (+)- and (−)-K252a". J Am Chem Soc 117 (41): 10413–4. 1995. doi:10.1021/ja00146a039.
 |
