Chemistry:Lead(II) laurate
From HandWiki
| |
| Names | |
|---|---|
| Other names
Lead(II) dodecanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
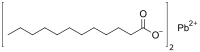
| Pb(C11H23COO)2[1] | |
| Molar mass | 606 |
| Appearance | White solid |
| Melting point | 104.7 °C (220.5 °F; 377.8 K) |
| Insoluble | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Lead(II) laurate is an metal-organic compound with the chemical formula Pb(O
2C(CH
2)
10CH
3)
2. It is classified as a metallic soap, i.e. a metal derivative of a fatty acid. Like most soaps, it does not dissolve in water.[2][3] Lead soaps have been used as stabilizers and plasticizers in PVC.[4]
Preparation
Lead soaps are usually prepared by combining lead(II) oxide with molten fatty acid. An idealized equation is:
- PbO + RCO
2H → Pb(O
2CR)
2 + H
2O
In reality, lead soaps have complex formulas.
References
- ↑ Substances, United States Environmental Protection Agency Office of Toxic (May 1979) (in en). Toxic Substances Control Act. U.S. Government Printing Office. p. Volume III, p. 861. https://books.google.com/books?id=PCwBQIhLMrsC&dq=lead(ii)+laurate&pg=PA861. Retrieved 23 January 2023.
- ↑ (in en) Official Gazette of the United States Patent and Trademark Office: Patents. U.S. Department of Commerce, Patent and Trademark Office. 1985. p. 1839. https://books.google.com/books?id=ReKYrYL-z7QC&dq=Lead(II)+laurate&pg=PA1839. Retrieved 23 January 2023.
- ↑ (in en) Official Gazette of the United States Patent Office. The Office. 1967. p. 310. https://books.google.com/books?id=eg7hI_hnpc4C&dq=Lead(II)+laurate&pg=PA310. Retrieved 23 January 2023.
- ↑ Nora, Angelo; Szczepanek, Alfred; Koenen, Gunther (2001). "Metallic Soaps". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a16_361. ISBN 3527306730.
 |

