Chemistry:Lead polonide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| PbPo | |
| Molar mass | 416 g·mol−1 |
| Appearance | black crystals |
| Density | 9.64 g·cm−3[1] |
| Melting point | 550–630 °C(decomposes)[2] |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lead polonide is the polonide of lead, with the chemical formula of PbPo. It occurs naturally, as lead is produced in the alpha decay of polonium.[3]
Preparation
Lead polonide can be formed by reacting polonium vapour and lead under a vacuum.[4]
Properties
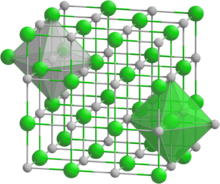
Lead polonide has a sodium chloride structure, which is the same as lead telluride. It has a cubic crystal structure, with the space group Fm3m (No. 225), with lattice constant a = 6.59 Å.[5]
References
- ↑ Harvey V. Moyer (1956), Chemical Properties of Polonium, pp. 96, doi:10.2172/4367751
- ↑ Terumitsu Miura, Toru Obara, Hiroshi Sekimoto (Nov 2007), "Experimental verification of thermal decomposition of lead polonide", Annals of Nuclear Energy 34 (11): 926–930, doi:10.1016/j.anucene.2007.05.009
- ↑ Weigel, F. (1959). "Chemie des Poloniums". Angewandte Chemie 71 (9): 289–316. doi:10.1002/ange.19590710902. Bibcode: 1959AngCh..71..289W.
- ↑ A. P. Hagen (Sep 2009), Inorganic Reactions and Methods, The Formation of Bonds to Group VIB (O, S, Se, Te, Po) Elements, John Wiley & Sons, pp. 161, ISBN 978-0470145401
- ↑ Richard Dalven (Dec 1973), Recent Studies Of Lead Polonide (PbPo), Lawrence Berkeley National Laboratory (Link )
 |
