Chemistry:Lithocholic acid
| |
| Names | |
|---|---|
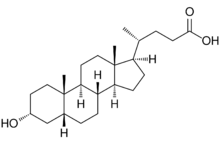
| IUPAC name
3α-Hydroxy-5β-cholan-24-oic acid
| |
| Systematic IUPAC name
(4R)-4-[(1R,3aS,3bR,5aR,7R,9aS,9bS,11aR)-7-Hydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]pentanoic acid | |
| Other names
Lithocholate; Lithocolic acid; 3α-Hydroxy-5β-cholanic acid; 5β-Cholan-24-oic acid-3α-ol
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3217757 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| C24H40O3 | |
| Molar mass | 376.57 g/mol |
| Melting point | 183 to 188 °C (361 to 370 °F; 456 to 461 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lithocholic acid, also known as 3α-hydroxy-5β-cholan-24-oic acid or LCA, is a bile acid that acts as a detergent to solubilize fats for absorption. Bacterial action in the colon produces LCA from chenodeoxycholic acid by reduction of the hydroxyl functional group at carbon-7 in the "B" ring of the steroid framework.[citation needed]
It has been implicated in human and experimental animal carcinogenesis.[2][3] Preliminary in vitro research suggests that LCA selectively kills neuroblastoma cells, while sparing normal neuronal cells and is cytotoxic to numerous other malignant cell types at physiologically relevant concentrations.[4]
Dietary fiber can bind to lithocholic acid and aid in its excretion in stool;[5] as such, fiber can protect against colon cancer.
LCA (and LCA acetate and LCA propionate) can activate the vitamin D receptor without raising calcium levels as much as vitamin D itself.[6]
LCA binds with high affinity (20 μM) to the human membrane enzyme NAPE-PLD, enhancing dimer assembly and enabling catalysis. NAPE-PLD catalyzes the release of anandamide and other N-acylethanolamines (NAE) from the membrane precursor N-acyl-phosphatidylethanolamine(NAPE). NAPE-PLD facilitates crosstalk between bile acid signals and lipid amide signals.[7] [8] [9]
LCA was also shown to have anti-aging effects in a yeast study.[10][11] A later study showed that the bile acid accumulates in the inner and outer mitochondrial membranes, altering the mitochondria's lipid composition by promoting or inhibiting various enzymes.[12]
References
- ↑ Lithocholic acid at Sigma-Aldrich
- ↑ Kozoni, V.; Tsioulias, G; Shiff, S; Rigas, B (2000). "The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: Differential effect on apoptosis in the presence of a colon carcinogen". Carcinogenesis 21 (5): 999–1005. doi:10.1093/carcin/21.5.999. PMID 10783324.
- ↑ Zeng H, Umar S, Rust B, Lazarova D, Bordonaro M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int J Mol Sci. 2019 Mar 11;20(5):1214. doi:10.3390/ijms20051214 PMID 30862015
- ↑ Goldberg, AA; Beach, A; Davies, GF; Harkness, TA; Leblanc, A; Titorenko, VI (2011). "Lithocholic bile acid selectively kills neuroblastoma cells, while sparing normal neuronal cells". Oncotarget 2 (10): 761–82. doi:10.18632/oncotarget.338. PMID 21992775.
- ↑ Jenkins, DJ; Wolever, TM; Rao, AV; Hegele, RA; Mitchell, SJ; Ransom, TP; Boctor, DL; Spadafora, PJ et al. (1993). "Effect on blood lipids of very high intakes of fiber in diets low in saturated fat and cholesterol". The New England Journal of Medicine 329 (1): 21–6. doi:10.1056/NEJM199307013290104. PMID 8389421.
- ↑ Ishizawa, M.; Matsunawa, M.; Adachi, R.; Uno, S.; Ikeda, K.; Masuno, H.; Shimizu, M.; Iwasaki, K.-i. et al. (2008). "Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia". The Journal of Lipid Research 49 (4): 763–772. doi:10.1194/jlr.M700293-JLR200. PMID 18180267.
- ↑ "Structure of Human N-Acylphosphatidylethanolamine-Hydrolyzing Phospholipase D: Regulation of Fatty Acid Ethanolamide Biosynthesis by Bile Acids". Structure 23 (3): 598–604. Dec 2014. doi:10.1016/j.str.2014.12.018. PMID 25684574.
- ↑ "Bile Acids as Enzyme Regulators". Chemistry & Biology 22 (4): 427–428. 2015. doi:10.1016/j.chembiol.2015.04.007.
- ↑ "Bile Acid Recognition by NAPE-PLD.". ACS Chem Biol 11 (10): 2908–2914. Oct 2016. doi:10.1021/acschembio.6b00624. PMID 27571266.
- ↑ Goldberg, AA; Richard, VR; Kyryakov, P; Bourque, SD; Beach, A; Burstein, MT; Glebov, A; Koupaki, O et al. (2010). "Chemical genetic screen identifies lithocholic acid as an anti-aging compound that extends yeast chronological life span in a TOR-independent manner, by modulating housekeeping longevity assurance processes". Aging 2 (7): 393–414. doi:10.18632/aging.100168. PMID 20622262.
- ↑ Arlia-Ciommo A, Leonov A, Mohammad K, Beach A, Richard VR, Bourque SD, Burstein MT, Goldberg AA, Kyryakov P, Gomez-Perez A, Koupaki O, Titorenko VI. Mechanisms through which lithocholic acid delays yeast chronological aging under caloric restriction conditions. Oncotarget. 2018 Oct 9;9(79):34945-34971. doi:10.18632/oncotarget.26188 PMID 30405886
- ↑ Beach, A.; Richard, V.R.; Leonov, A.; Burstein, M.T.; Bourque, S.D.; Koupaki, O.; Juneau, M.; Feldman, R.; Iouk, T.; Titorenko, V.I. Mitochondrial membrane lipidome defines yeast longevity. Aging 2013, 5, 551–574.
 |

