Chemistry:Lobry de Bruyn–van Ekenstein transformation
In carbohydrate chemistry, the Lobry de Bruyn–van Ekenstein transformation also known as the Lobry de Bruyn–Alberda–van Ekenstein transformation is the base or acid catalyzed transformation of an aldose into the ketose isomer or vice versa, with a tautomeric enediol as reaction intermediate. Ketoses may be transformed into 3-ketoses, etcetera. The enediol is also an intermediate for the epimerization of an aldose or ketose. [1] [2]
The reactions are usually base catalyzed, but can also take place under acid or neutral conditions.[1] A typical rearrangement reaction is that between the aldose glyceraldehyde and the ketose dihydroxyacetone in a chemical equilibrium.
The Lobry de Bruyn–van Ekenstein transformation is relevant for the industrial production of certain ketoses and was discovered in 1885 by Cornelis Adriaan Lobry van Troostenburg de Bruyn and Willem Alberda van Ekenstein.[3][4][5][6][7][8]
Aldose-ketose transformation
The following scheme describes the interconversion between an aldose and a ketose, where R is any organic residue.
The equilibrium or the reactant to product ratio depends on concentration, solvent, pH and temperature. At equilibrium the aldose and ketose form a mixture which in the case of the glyceraldehyde and dihydroxyacetone is also called glycerose.
A related reaction is the alpha-ketol rearrangement.
Epimerization
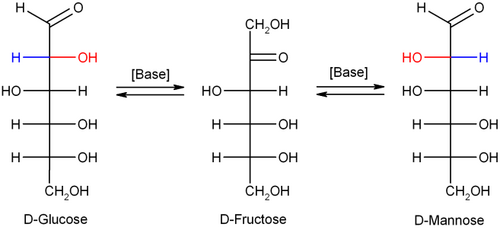
The carbon atom at which the initial deprotonation takes place is a stereocenter. If, for example, D-glucose (an Aldose) rearranges to D-fructose, the ketose, the stereochemical configuration is lost in the enol form. In the chemical reaction the enol can be protonated from two faces, resulting in the backformation of glucose or the formation of the epimer D-mannose. The final product is a mix of D-glucose, D-fructose and D-mannose.
References
- ↑ 1.0 1.1 Momcilo Miljkovic Carbohydrates: Synthesis, Mechanisms, and Stereoelectronic Effects 2009 (Google books)
- ↑ ANGYAL, S.J.: The Lobry de Bruyn–Alberda van Ekenstein transformation and related reactions, in: Glycoscience: epimerisation, isomerisation and rearrangement reactions of carbohydrates, Vol. 215, (Ed.: STÜTZ, A.E.), Springer-Verlag, Berlin, 2001, 1–14
- ↑ de Bruyn, C. A. Lobry (1895). "Action des alcalis dilués sur les hydrates de carbone I. (Expériences provisoires)" (in fr). Recueil des Travaux Chimiques des Pays-Bas 14 (6): 156-165. doi:10.1002/recl.18950140602.
- ↑ de Bruyn, C. A. Lobry; van Ekenstein, W. Alberda (1895). "Action des alcalis sur les sucres, II. Transformation réciproque des uns dans les autres des sucres glucose, fructose et mannose" (in fr). Recueil des Travaux Chimiques des Pays-Bas 14 (7): 203-216. doi:10.1002/recl.18950140703.
- ↑ de Bruyn, C. A. Lobry; van Ekenstein, W. Alberda (1896). "Action des alcalis sur les sucres, III. Transformation des sucres sous l'influence de l'hydroxyde de plomb" (in fr). Recueil des Travaux Chimiques des Pays-Bas 15 (3): 92-96. doi:10.1002/recl.18960150306.
- ↑ de Bruyn, C. A. Lobry; van Ekenstein, W. Alberda (1897). "Action des alcalis sur les sucres. IV: Remarques générales" (in fr). Recueil des Travaux Chimiques des Pays-Bas et de la Belgique 16 (8): 257-261. doi:10.1002/recl.18970160805.
- ↑ de Bruyn, C. A. Lobry; van Ekenstein, W. Alberda (1897). "Action des alcalis sur les sucres. V: Transformation de la galactose. Les tagatoses, et la galtose" (in fr). Recueil des Travaux Chimiques des Pays-Bas et de la Belgique 16 (9): 262-273. doi:10.1002/recl.18970160902.
- ↑ de Bruyn, C. A. Lobry; van Ekenstein, W. Alberda (1897). "Action des alcalis sur les sucres. VI: La glutose et la pseudo‐fructose" (in fr). Recueil des Travaux Chimiques des Pays-Bas et de la Belgique 16 (9): 274-281. doi:10.1002/recl.18970160903.