Chemistry:Losmapimod
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
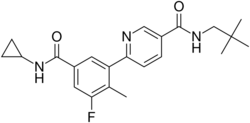
| Formula | C22H26FN3O2 |
| Molar mass | 383.467 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Losmapimod (GW856553X) is an investigational drug being developed by Fulcrum Therapeutics for the treatment of facioscapulohumeral muscular dystrophy (FSHD); a phase III clinical trial is pending approval. Losmapimod selectively inhibits enzymes p38α/β mitogen-activated protein kinases (MAPKs), which are modulators of DUX4 expression and mediators of inflammation.[1]
Potential treatment for FSHD
Fulcrum Therapeutics, a Massachusetts-based biotechnology company, identified p38α/β MAPK inhibitors as potent suppressors of DUX4 expression, the de-suppression of which is accepted as the cause FSHD. Among p38α/β MAPK inhibitors, Fulcrum chose losmapimod as its preferred developmental candidate due to its "substantial and attractive preclinical and clinical data" from previous GlaxoSmithKline (GSK) clinical trials.[2]
As Fulcrum discovered the potential utility of p38 MAPK inhibition in treating FSHD, a Saint Louis University (SLU) research group independently arrived at the same conclusion.[3][4] The SLU research group found that p38α and p38β isoforms independently contribute to DUX4 expression, which indicates potential gain in exploring isoform specific (p38α or p38β) inhibition to balance therapeutic effects with side effects.
A theoretical limitation of losmapimod is that p38 kinase inhibition could impair myogenesis, the opposite effect of what is desired. Facio Therapies, a Dutch pharmaceutical company with their own drug candidate for FSHD, announced that they had eliminated p38 kinase inhibitors (including losmapimod) as a developmental candidate because p38 kinase inhibitors resulted in impaired myotube formation on their drug development platform.[5][6] Indeed, others have found that p38α abrogation impairs myotube formation.[7] However, Fulcrum found that p38 kinase inhibition did not impair myotube fusion at levels sufficient for DUX4 reduction.[8]
Fulcrum development timeline
- March 2022: Fulcrum announced plan for a phase III clinical trial for treatment of FSHD, named REACH.[9] Unlike the previous trial, the primary endpoint will be reachable workspace.[9]
- June 2021: Results of a randomized controlled phase IIb clinical trial, named ReDUX4, showed statistically significant slowing of muscle function deterioration, albeit biomarkers were unchanged. Further trials are pending.[10][11][12][13][14]
- March 2021: Fulcrum announced discontinuation of LOSVID due to challenges with enrollment and the rapidly evolving environment of COVID-19 treatment.[15]
- June 2020: Fulcrum announced applying to the FDA to initiate a phase III clinical trial, LOSVID, for losmapimod in treatment of COVID-19. Recent evidence indicates that p38 MAPK inhibition could be therapeutic, possibly by attenuating the exaggerated inflammatory response following SARS-CoV-2 infection.[16]
- January 2020: Fulcrum announced receiving orphan drug status for losmapimod.
- October 2019: Fulcrum announced preliminary results of their phase 1 clinical trial of losmapimod. Oral dosing of losmapimod demonstrated sustained muscle tissue drug concentrations that in preclinical in vitro studies had shown effective in reducing DUX4 levels.[17]
- April 2019: Fulcrum acquired from GSK the global rights to losmapimod.[18]
Historical Investigations
Losmapimod was discovered and unsuccessfully developed by GSK for treating multiple medical conditions. Despite failing to prove efficacy, GSK clinical trials showed that losmapimod is generally well tolerated across more than 3,500 subjects.[2][19][20]
GSK investigated losmapimod as a therapeutic for patients post-myocardial infarction (heart attack). Despite phase II clinical trials[21][22][23][24][25][26] the phase IIIA clinical trial (LATITUDE)[27] failed to show significantly improved clinical outcomes.[28] In October 2015 GSK announced cancelling the planned phase IIIB trial, but would "evaluate all options for future development."[29]
GSK investigated losmapimod as a therapeutic for COPD, but multiple phase II clinical trials[30][31][32] failed to show that losmapimod improves exercise tolerance,[19] lung function,[19] arterial inflammation,[20] endothelial function,[20] or rate of COPD exacerbations[33] in subjects with COPD. GSK terminated development of losmapimod for COPD in 2016.[34][35]
GSK investigated losmapimod as a therapeutic for major depressive disorder (MDD) on the basis of depression being correlated with elevated pro-inflammatory cytokines.[36] Phase II clinical trials[37][38] failed to show a significant improvement in depression symptoms and biomarkers.[36]
References
- ↑ "p38alpha mitogen-activated protein kinase inhibitors: optimization of a series of biphenylamides to give a molecule suitable for clinical progression". Journal of Medicinal Chemistry 52 (20): 6257–69. October 2009. doi:10.1021/jm9004779. PMID 19772287.
- ↑ 2.0 2.1 "FORM S-1 REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933 FULCRUM THERAPEUTICS, INC.". Securities and Exchange Commission. https://www.sec.gov/Archives/edgar/data/1680581/000104746919004014/a2239182zs-1a.htm.
- ↑ "Clinically Advanced p38 Inhibitors Suppress DUX4 Expression in Cellular and Animal Models of Facioscapulohumeral Muscular Dystrophy". The Journal of Pharmacology and Experimental Therapeutics 370 (2): 219–230. August 2019. doi:10.1124/jpet.119.259663. PMID 31189728.
- ↑ "In Lab, SLU Research Halts Toxic Protein Linked to Muscular Dystrophy". www.newswise.com (Newswise). 23 October 2019. https://www.newswise.com/articles/in-lab-slu-research-halts-toxic-protein-linked-to-muscular-dystrophy.
- ↑ "Facio to present at the World Muscle Society Congress". Facio Therapies. 30 September 2019. https://www.facio-therapies.com/news/facio-to-present-at-the-world-muscle-society-congress/.
- ↑ "Facio reveals novel mechanism targeting the cause of FSHD". Facio Therapies. 24 June 2019. https://www.facio-therapies.com/news/facio-reveals-novel-mechanism-targeting-the-cause-of-fshd/.
- ↑ "Genetic analysis of p38 MAP kinases in myogenesis: fundamental role of p38alpha in abrogating myoblast proliferation". The EMBO Journal 26 (5): 1245–56. March 2007. doi:10.1038/sj.emboj.7601587. PMID 17304211.
- ↑ "P38α Regulates Expression of DUX4 in Facioscapulohumeral Muscular Dystrophy" (in en). bioRxiv: 700195. 12 July 2019. doi:10.1101/700195. https://www.biorxiv.org/content/10.1101/700195v1. Retrieved 9 November 2019.
- ↑ 9.0 9.1 "Fulcrum announces Phase 3 trial of losmapimod". FSHD Society. 3 March 2022. https://www.fshdsociety.org/2022/03/03/fulcrum-announces-phase-3-trial-of-losmapimod/?fbclid=IwAR3v90tI6S9_0116ZsQKQKLXfDI0FQ-sH9gvW43Kcd-PYxaGQ9iCL-cTotg.
- ↑ "ReDUX4 trial result exceeds expectations". Wayback Machine. 2021-06-24. https://www.fshdsociety.org/2021/06/24/redux4-trial-result-exceeds-expectations/.
- ↑ "Efficacy and Safety of Losmapimod in Subjects With Facioscapulohumeral Muscular Dystrophy (FSHD)" (in en). United States National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04003974?term=losmapimod&rank=3.
- ↑ Clinical trial number NCT04003974 for "Efficacy and Safety of Losmapimod in Subjects With Facioscapulohumeral Muscular Dystrophy (FSHD) (FSHD)" at ClinicalTrials.gov
- ↑ "Fulcrum's losmapimod fails interim analysis in muscle wasting trial" (in en). FierceBiotech. https://www.fiercebiotech.com/biotech/fulcrum-s-losmapimod-fails-interim-analysis-muscle-wasting-trial.
- ↑ Clinical trial number NCT04004000 for "Evaluation of Safety, Tolerability, and Changes in Biomarker and Clinical Outcome Assessments of Losmapimod for FSHD1" at ClinicalTrials.gov
- ↑ Inc, Fulcrum Therapeutics (4 March 2021). "Fulcrum Therapeutics Reports Recent Business Highlights and Fourth Quarter and Full Year 2020 Financial Results" (in en). GlobeNewswire News Room. https://www.globenewswire.com/news-release/2021/03/04/2186976/0/en/Fulcrum-Therapeutics-Reports-Recent-Business-Highlights-and-Fourth-Quarter-and-Full-Year-2020-Financial-Results.html.
- ↑ "Losmapimod as potential COVID-19 treatment?". 10 June 2020. https://www.fshdsociety.org/2020/06/10/losmapimod-as-potential-covid-19-treatment/?fbclid=IwAR1pemUbJa76klnqVhwl-mHvGWwnjChlrL-4lm6y8YqpSgJx3d_1TbBZ60g.
- ↑ "Fulcrum Therapeutics Announced Results of Phase 1 Clinical Trial of Losmapimod in FSHD". GlobeNewswire News Room. 4 October 2019. https://www.globenewswire.com/news-release/2019/10/04/1925160/0/en/Fulcrum-Therapeutics-Announced-Results-of-Phase-1-Clinical-Trial-of-Losmapimod-in-FSHD.html.
- ↑ "Fulcrum Therapeutics Acquires Global Rights to Losmapimod, a Potential Disease-Modifying Therapy for Facioscapulohumeral Muscular Dystrophy". BioSpace. 23 April 2019. https://www.biospace.com/article/releases/fulcrum-therapeutics-acquires-global-rights-to-losmapimod-a-potential-disease-modifying-therapy-for-facioscapulohumeral-muscular-dystrophy/.
- ↑ 19.0 19.1 19.2 "Efficacy and safety of the p38 MAPK inhibitor losmapimod for patients with chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial". The Lancet. Respiratory Medicine 2 (1): 63–72. January 2014. doi:10.1016/S2213-2600(13)70200-5. PMID 24461903.
- ↑ 20.0 20.1 20.2 "The p38 mitogen activated protein kinase inhibitor losmapimod in chronic obstructive pulmonary disease patients with systemic inflammation, stratified by fibrinogen: A randomised double-blind placebo-controlled trial". PLOS ONE 13 (3): e0194197. 2018. doi:10.1371/journal.pone.0194197. PMID 29566026. Bibcode: 2018PLoSO..1394197F.
- ↑ Clinical trial number NCT00474864 for "Study To Evaluate The Effects Of GW856553 On Endothelial Function/Vascular Compliance In Subjects With Dyslipidaemia." at ClinicalTrials.gov
- ↑ Clinical trial number NCT00633022 for "A Study to Evaluate the Effects of 3 Months Dosing With GW856553, as Assessed FDG-PET/CT Imaging" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00910962 for "Study to Evaluate the Safety of 12 Weeks of Dosing With GW856553 and Its Effects on Inflammatory Markers, Infarct Size, and Cardiac Function in Subjects With Myocardial Infarction Without ST-segment Elevation (Solstice)" at ClinicalTrials.gov
- ↑ "Inhibition of p38 mitogen-activated protein kinase improves nitric oxide-mediated vasodilatation and reduces inflammation in hypercholesterolemia". Circulation 123 (5): 515–23. February 2011. doi:10.1161/CIRCULATIONAHA.110.971986. PMID 21262998.
- ↑ "Effects of p38 mitogen-activated protein kinase inhibition on vascular and systemic inflammation in patients with atherosclerosis". JACC. Cardiovascular Imaging 5 (9): 911–22. September 2012. doi:10.1016/j.jcmg.2012.02.016. PMID 22974804.
- ↑ "Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-ST-segment elevation myocardial infarction: a randomised phase 2 trial". Lancet 384 (9949): 1187–95. September 2014. doi:10.1016/S0140-6736(14)60417-7. PMID 24930728.
- ↑ Clinical trial number NCT02145468 for "A Phase 3 Clinical Outcomes Study to Compare the Incidence of Major Adverse Cardiovascular Events in Subjects Presenting With Acute Coronary Syndrome Treated With Losmapimod Compared to Placebo (LATITUDE-TIMI 60)" at ClinicalTrials.gov
- ↑ "Effect of Losmapimod on Cardiovascular Outcomes in Patients Hospitalized With Acute Myocardial Infarction: A Randomized Clinical Trial". JAMA 315 (15): 1591–9. April 2016. doi:10.1001/jama.2016.3609. PMID 27043082.
- ↑ "GSK provides update on LATITUDE-TIMI 60 (losmapimod cardiovascular study)". GSK. 27 October 2015. https://www.gsk.com/en-gb/media/press-releases/gsk-provides-update-on-latitude-timi-60-losmapimod-cardiovascular-study/.
- ↑ Clinical trial number NCT01218126 for "Randomised, Double-Blind, Placebo-Controlled, Parallel-Group, Multi-centre, Dose Ranging Study to Evaluate the Efficacy and Safety of Losmapimod Tablets Administered Twice Daily Compared With Placebo for 24 Weeks in Adult Subjects With Chronic Obstructive Pulmonary Disease (COPD)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT01541852 for "Losmapimod in Chronic Obstructive Pulmonary Disease Patients Stratified by Fibrinogen. (EVOLUTION)" at ClinicalTrials.gov
- ↑ Clinical trial number NCT02299375 for "Safety and Efficacy Study of Losmapimod (GW856553) in Frequently Exacerbating Participants With Chronic Obstructive Pulmonary Disease (COPD)" at ClinicalTrials.gov
- ↑ "Biological effects of p38 MAPK inhibitor losmapimod does not translate to clinical benefits in COPD". Respiratory Medicine 130: 20–26. September 2017. doi:10.1016/j.rmed.2017.07.002. PMID 29206629.
- ↑ Keown, Alex (Oct 26, 2016). "GlaxoSmithKline Terminates Development of Losmapimod for COPD". BioSpace. https://www.biospace.com/article/glaxosmithkline-terminates-development-of-losmapimod-for-copd-/.
- ↑ Lawrence, Stacy (Oct 26, 2016). "GSK drops a pair of late-stage candidates in COPD, HIV" (in en). FierceBiotech. https://www.fiercebiotech.com/biotech/gsk-drops-a-pair-late-stage-candidates-copd-hiv.
- ↑ 36.0 36.1 "Evaluation of antidepressant properties of the p38 MAP kinase inhibitor losmapimod (GW856553) in Major Depressive Disorder: Results from two randomised, placebo-controlled, double-blind, multicentre studies using a Bayesian approach". Journal of Psychopharmacology 28 (6): 570–81. June 2014. doi:10.1177/0269881114529377. PMID 24699061.
- ↑ Clinical trial number NCT00569062 for "A Study of GW856553X For the Treatment of Depression" at ClinicalTrials.gov
- ↑ Clinical trial number NCT00976560 for "Clinical Study to Test a New Drug to Treat Major Depression" at ClinicalTrials.gov
 |
