Chemistry:Lotaustralin
From HandWiki
| |
| |
| Names | |
|---|---|
| IUPAC name
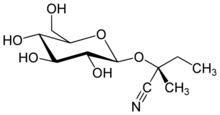
(2R)-2-(β-D-Glucopyranosyloxy)-2-methylbutanenitrile
| |
| Systematic IUPAC name
(2R)-2-Methyl-2-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butanenitrile | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H19NO6 | |
| Molar mass | 261.27 g/mol |
| Appearance | colorless needles |
| Density | 1.36 g·cm−3 |
| Melting point | 139 °C (282 °F; 412 K)[1] |
| good, also good in Ethyl acetate[1] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lotaustralin is a cyanogenic glucoside found in small amounts in Fabaceae austral trefoil (Lotus australis),[1] cassava (Manihot esculenta), lima bean (Phaseolus lunatus),[2] roseroot (Rhodiola rosea)[3] and white clover (Trifolium repens),[4] among other plants. Lotaustralin is the glucoside of methyl ethyl ketone cyanohydrin and is structurally related to linamarin, the acetone cyanohydrin glucoside also found in these plants. Both lotaustralin and linamarin may be hydrolyzed by the enzyme linamarase to form glucose and a precursor to the toxic compound hydrogen cyanide.
References
- ↑ 1.0 1.1 1.2 Shmuel Yannai: Dictionary of Food Compounds with CD-ROM: Additives, Flavors, and Ingredients. CRC Press, 2003, ISBN 978-1-58488-416-3, p. 688
- ↑ "Pattern of the Cyanide-Potential in Developing Fruits : Implications for Plants Accumulating Cyanogenic Monoglucosides (Phaseolus lunatus) or Cyanogenic Diglucosides in Their Seeds (Linum usitatissimum, Prunus amygdalus)". Plant Physiol 94 (1): 28–34. 1990. doi:10.1104/pp.94.1.28. PMID 16667698.
- ↑ "Lotaustralin from Rhodiola rosea roots". Fitoterapia 75 (6): 612–4. 2004. doi:10.1016/j.fitote.2004.06.002. PMID 15351122.
- ↑ "Notes on poisoning: Trifolium repens". Canadian Poisonous Plants Information System. May 30, 2006. http://www.cbif.gc.ca/pls/pp/ppack.info?p_psn=258&p_type=all&p_sci=sci&p_x=pp.
 |
