Chemistry:Methacrylic anhydride
From HandWiki
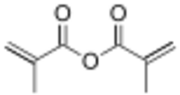
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylprop-2-enoic anhydride | |
| Other names
Methacrylic anhydride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H10O3 | |
| Molar mass | 154.165 g·mol−1 |
| Density | 1.035 g/cm3 |
| Boiling point | 87 °C/13 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Methacrylic anhydride is a liquid which reacts with water exothermically.[1]
It can also react with hydroxyl- and amino-groups present in some organic compounds leading to covalent attachment of methacryloyl moieties. These functional groups could be successfully used either in subsequent polymerisation or reactions with thiols. For example, chitosan modified by methacryloylation exhibits better ability to adhere to mucosal surfaces (mucoadhesion) due to its reactivity with thiols groups present in cysteine domains of mucins ).[2]
Uses
Methacrylic anhydride is used as an intermediate agent in plastics material and resin manufacturing. [3]
References
- ↑ Methacrylic anhydride at www.chemicalbook.com.
- ↑ Kolawole, Oluwadamilola M.; Lau, Wing Man; Khutoryanskiy, Vitaliy V. (2018-10-25). "Methacrylated chitosan as a polymer with enhanced mucoadhesive properties for transmucosal drug delivery". International Journal of Pharmaceutics 550 (1): 123–129. doi:10.1016/j.ijpharm.2018.08.034. ISSN 0378-5173. PMID 30130604. http://centaur.reading.ac.uk/78982/1/methacrylated%20chitosan%20for%20intravesical%20delivery-accepted.pdf.
- ↑ "Methacrylic anhydride". National Institute of Health. https://pubchem.ncbi.nlm.nih.gov/compound/Methacrylic-anhydride#section=General-Manufacturing-Information. Retrieved 2023-01-20.
 |

