Chemistry:Methanesulfonyl chloride
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methanesulfonyl chloride | |||
| Other names
Mesyl chloride
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
| |||
| Properties | |||
| CH 3SO 2Cl | |||
| Molar mass | 114.54 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Odor | Pungent, unpleasant[1] | ||
| Density | 1.480 g/cm3 | ||
| Melting point | −32 °C (−26 °F; 241 K)[2] | ||
| Boiling point | 161 °C (322 °F; 434 K) (at 730 mmHg) | ||
| Reacts[3][4] | |||
| Solubility | Soluble in alcohol, ether and most organic solvents[5] | ||
| Hazards | |||
| Main hazards | Lachrymator, highly toxic, corrosive | ||
| Flash point | >110 °C (230 °F; 383 K)[6] | ||
| Related compounds | |||
Other anions
|
Methanesulfonyl fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
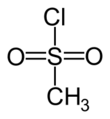
Methanesulfonyl chloride (mesyl chloride) is an organosulfur compound with the formula CH
3SO
2Cl. Using the organic pseudoelement symbol Ms for the methanesulfonyl (or mesyl) group CH
3SO
2–, it is frequently abbreviated MsCl in reaction schemes or equations. It is a colourless liquid that dissolves in polar organic solvents but is reactive toward water, alcohols, and many amines. The simplest organic sulfonyl chloride, it is used to make methanesulfonates and to generate the elusive molecule sulfene (methylenedioxosulfur(VI)).[7]
Preparation
It is manufactured by the reaction of methane and sulfuryl chloride in a radical reaction:
- CH
4 + SO
2Cl
2 → CH
3SO
2Cl + HCl
Another method of manufacture entails chlorination of methanesulfonic acid with thionyl chloride or phosgene:
- CH
3SO
3H + SOCl
2 → CH
3SO
2Cl + SO
2 + HCl - CH
3SO
3H + COCl
2 → CH
3SO
2Cl + CO
2 + HCl
Reactions
Methanesulfonyl chloride is a precursor to many compounds because it is highly reactive. It is an electrophile, functioning as a source of the "CH3SO2+" group.[7][clarification needed]
Methanesulfonates
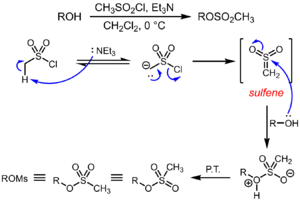
Methanesulfonyl chloride is mainly used to give methanesulfonates by its reaction with alcohols in the presence of a non-nucleophilic base.[8] In contrast to the formation of toluenesulfonates from alcohols and p-toluenesulfonyl chloride in the presence of pyridine, the formation of methanesulfonates is believed to proceed via a mechanism wherein methanesulfonyl chloride first undergoes an E1cb elimination to generate the highly reactive parent sulfene (CH
2=SO
2), followed by attack by the alcohol and rapid proton transfer to generate the observed product. This mechanistic proposal is supported by isotope labeling experiments and the trapping of the transient sulfene as cycloadducts.[9]
Methanesulfonates are used as intermediates in substitution reactions, elimination reactions, reductions, and rearrangement reactions. When treated with a Lewis acid, oxime methanesulfonates undergo facile Beckmann rearrangement.[10]
Methanesulfonates are occasionally used as a protecting group for alcohols. They are stable to acidic conditions and is cleaved back to the alcohol using sodium amalgam.[11]
Methanesulfonamides
Methanesulfonyl chloride react with primary and secondary amines to give methanesulfonamides. Unlike methanesulfonates, methanesulfonamides are very resistant toward hydrolysis under both acidic and basic conditions.[7] When used as a protecting group, they can be converted back to amines using lithium aluminium hydride or a dissolving metal reduction.[12]
Addition to alkynes
In the presence of copper(II) chloride, methanesulfonyl chloride will add across alkynes to form β-chloro sulfones.[13]
Formation of heterocycles
Upon treatment with a base, such as triethylamine, methanesulfonyl chloride will undergo an elimination to form sulfene. Sulfene can undergo cycloadditions to form various heterocycles. α-Hydroxyketones react with sulfene to form five-membered sultones.[14]
Miscellaneous
Forming acyliminium ions from α-hydroxyamides can be done using methanesulfonyl chloride and a base, typically triethylamine.[15]
Safety
Methanesulfonyl chloride is highly toxic by inhalation, corrosive, and acts as a lachrymator. It reacts with nucleophilic reagents (including water) in a strongly exothermic manner. When heated to decomposition point, it emits toxic vapors of sulfur oxides and hydrogen chloride.[16]
References
- ↑ "Methanesulfonyl chloride". https://pubchem.ncbi.nlm.nih.gov/compound/Methanesulfonyl-chloride#section=Physical-Description.
- ↑ "Methanesulfonyl chloride". https://pubchem.ncbi.nlm.nih.gov/compound/Methanesulfonyl-chloride#section=Decomposition.
- ↑ cameochemicals.noaa.gov/chemical/11835
- ↑ "MSDS". Archived from the original on 2005-04-30. https://web.archive.org/web/20050430205030/http://www.conncoll.edu/offices/envhealth/MSDS/chemistry/M/methanesulfonylchloride.htm. Retrieved 2013-01-14.
- ↑ "Methanesulfonyl chloride". https://pubchem.ncbi.nlm.nih.gov/compound/Methanesulfonyl-chloride#section=Solubility.
- ↑ "Methanesulfonyl chloride". https://pubchem.ncbi.nlm.nih.gov/compound/Methanesulfonyl-chloride#section=Decomposition.
- ↑ 7.0 7.1 7.2 Valerie Vaillancourt, Michele M. Cudahy, Matthew M. Kreilein and Danielle L. Jacobs "Methanesulfonyl Chloride" in E-EROS Encyclopedia for Reagents in Organic Synthesis. doi:10.1002/047084289X.rm070.pub2
- ↑ Furst, A.; Koller, F. (1947). "Über Steroide und Sexualhormone. Ein neuer Weg zur Herstellung der α-Oxyde von Cholesterin und trans-Dehydro-androsteron". Helv. Chim. Acta 30 (6): 1454–60. doi:10.1002/hlca.19470300609. PMID 20272042.
- ↑ King, James Frederick (1975-01-01). "Return of sulfenes". Accounts of Chemical Research 8 (1): 10–17. doi:10.1021/ar50085a002. ISSN 0001-4842.
- ↑ Maruoka, K.; Miyazaki, T.; Ando, M.; Matsumura, Y.; Sakane, S.; Hattori, K.; Yamamoto, H. (1983). "Organoaluminum-promoted Beckmann rearrangement of oxime sulfonates". J. Am. Chem. Soc. 105 (9): 2831. doi:10.1021/ja00347a052.
- ↑ Webster, K. T.; Eby, R.; Schuerch, C. (1983). "Selective demesylation of 2-O-(methylsulfonyl)-?-mannopyranoside derivatives with sodium amalgam and 2-propanol". Carbohydr. Res. 123 (2): 335. doi:10.1016/0008-6215(83)88490-0.
- ↑ Merlin, P.; Braekman, J. C.; Daloze, D. (1988). "Stereoselective synthesis of (±)-tetraponerine-8, a defence alkaloid of the ant Tetraponera sp". Tetrahedron Lett. 29 (14): 1691. doi:10.1016/S0040-4039(00)82019-5.
- ↑ Amiel, Y. (1971). "Addition of sulfonyl chlorides to acetylenes". Tetrahedron Lett. 12 (8): 661–663. doi:10.1016/S0040-4039(01)96524-4.
- ↑ Potonay, T.; Batta, G.; Dinya, Z. (1988). "Flavonoids. 41. Stereospecific synthesis of 2,3-dihydro-c-3-substituted-t-3-methyl-r-2-phenyl-4H-1-benzopyran-4-ones". Journal of Heterocyclic Chemistry 25: 343–347. doi:10.1002/jhet.5570250158.
- ↑ Chamberlin, A. R.; Nguyen, H. D.; Chung, J. Y. L. (1984). "Cationic cyclization of ketene dithioacetals. A general synthesis of pyrrolizidine, indolizidine, and quinolizidine alkaloid ring systems". J. Org. Chem. 49 (10): 1682. doi:10.1021/jo00184a002.
- ↑ "Methanesulfonyl chloride". https://pubchem.ncbi.nlm.nih.gov/compound/Methanesulfonyl-chloride#section=Decomposition.
 |